 3
3 D. There would likely not be life on Earth because its surface would be much colder and Earth's water would be frozen.
Explanation:
The greenhouse Gases in the Earth's atmosphere has the capability to let sun light run pass through the atmosphere, and trap the heat within the Earth after doing so.
This heat is what keep our current Earth had a livable temperature for most plants and animals.
Without the green houses gases, the average temperature in earth will be lowered and the vast majority of life forms that we currently know will most likely go extinct.
 3
3 D. There would likely not be life on Earth because its surface would be much colder and Earth's water would be frozen.
Explanation:
The greenhouse Gases in the Earth's atmosphere has the capability to let sun light run pass through the atmosphere, and trap the heat within the Earth after doing so.
This heat is what keep our current Earth had a livable temperature for most plants and animals.
Without the green houses gases, the average temperature in earth will be lowered and the vast majority of life forms that we currently know will most likely go extinct.
 1
1 The correct answer is D) There would likely not be life on Earth because its surface would be much colder and Earth's water would be frozen.
Life on Earth could be affected if the atmosphere did NOT contain greenhouse gases in the following way: "There would likely not be life on Earth because its surface would be much colder and Earth's water would be frozen."
Scientists agree that this is basically the importance of greenhouse gases on the atmosphere of planet earth. Without these gases, the surface of the earth would have an average temperature of minus 18 centigrades. Many life forms as we know them could not survive under this hairs climate condition. Furthermore, most of the surface of the earth would be covered on ice.
How does energy from the Sun travel to Earth?
Explanation:
How does energy from the Sun travel to Earth?
Explanation:
Answer:
AStep-by-step explanation:
The input force is 50 N. But it will not create not any change. No mechanical advantage is observed.
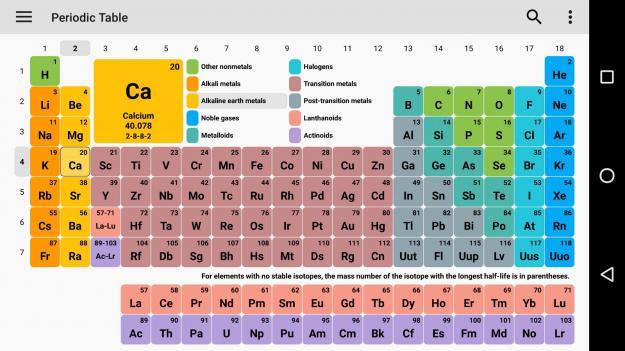
Calcium (Ca)(On the periodic table, ionization energy increases as you go up and to the right of the periodic table)
Answer:
Taking into accoun the ideal gas law, The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.
An ideal gas is a theoretical gas that is considered to be composed of point particles that move randomly and do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas. The universal constant of ideal gases R has the same value for all gaseous substances.
Explanation:
In this case, you know:
P= 0.884 atm
V= ?
n= 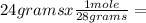 0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
R=0.082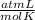
T= 328 K
Replacing in the ideal gas law:
0.884 atm×V= 0.857 moles× 0.082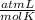 ×328 K
×328 K
Solving:
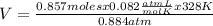
V= 26.07 L
The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.

It will provide an instant answer!
