 7
7 All scientific research helps to generate new knowledge and improve the understanding of what is currently known, so it is important to support all scientific progress as long as it is developed in safe conditions and with the aim of increasing the knowledge of the world that we surrounds and of the universe.
Explanation:
The super-heavy elements are a group of elements of the periodic table that have very large and therefore very heavy nuclei, because of this they are very unstable (sometimes they are only stable less than a second and then they decompose into other atoms more stable) and that is why they have not been discovered so far in nature.
Super-heavy elements are studied in special laboratories as they are created when other atoms of smaller nuclei are "stuck" through a shock that requires a lot of energy. This clash that a new element can generate is achieved after millions of tests are carried out, and in each attempt a lot of information is generated that must be analyzed by super computers in order to determinate whether that attempt was successful (created a new element) or not.
That is why it takes a long time to study and develop new elements, but during this time and through the tests we get very important information that allows us to better understand the behavior of atoms and ratify or assume new theories that advance in the scientific community which may not currently be applicable but in the future.
Thanks to the past investigations of scientist along the history they discovered all the elements of the current periodic table that allows us to undestand the properties of atoms and their uses for a huge amount of applications.
 7
7 All scientific research helps to generate new knowledge and improve the understanding of what is currently known, so it is important to support all scientific progress as long as it is developed in safe conditions and with the aim of increasing the knowledge of the world that we surrounds and of the universe.
Explanation:
The super-heavy elements are a group of elements of the periodic table that have very large and therefore very heavy nuclei, because of this they are very unstable (sometimes they are only stable less than a second and then they decompose into other atoms more stable) and that is why they have not been discovered so far in nature.
Super-heavy elements are studied in special laboratories as they are created when other atoms of smaller nuclei are "stuck" through a shock that requires a lot of energy. This clash that a new element can generate is achieved after millions of tests are carried out, and in each attempt a lot of information is generated that must be analyzed by super computers in order to determinate whether that attempt was successful (created a new element) or not.
That is why it takes a long time to study and develop new elements, but during this time and through the tests we get very important information that allows us to better understand the behavior of atoms and ratify or assume new theories that advance in the scientific community which may not currently be applicable but in the future.
Thanks to the past investigations of scientist along the history they discovered all the elements of the current periodic table that allows us to undestand the properties of atoms and their uses for a huge amount of applications.
 38
38 The correct answer is C) the establishment of a project in Los Alamos, New Mexico, to develop.
Based on the lesson, the event that was a direct result of Einstein’s letter was the establishment of a project in Los Alamos, New Mexico, to develop.
We are talking about the famous "Einstein-Szilard letter," that was written to the United States, President Franklin D. Roosevelt. It was Alexander Sachs -a close friend of President Roosevelt- who delivered the letter to him on October 11, 1939. The content of the letter left a big impression in Roosevelt's mind, he ordered the creation of an advisory Uranium Committee. Years later, on December 28, 1942, President Roosevelt ordered the creation of the Manhattan Project to create nuclear weapons.
 38
38 The correct answer is C) the establishment of a project in Los Alamos, New Mexico, to develop.
Based on the lesson, the event that was a direct result of Einstein’s letter was the establishment of a project in Los Alamos, New Mexico, to develop.
We are talking about the famous "Einstein-Szilard letter," that was written to the United States, President Franklin D. Roosevelt. It was Alexander Sachs -a close friend of President Roosevelt- who delivered the letter to him on October 11, 1939. The content of the letter left a big impression in Roosevelt's mind, he ordered the creation of an advisory Uranium Committee. Years later, on December 28, 1942, President Roosevelt ordered the creation of the Manhattan Project to create nuclear weapons.
Answer:
AStep-by-step explanation:
The input force is 50 N. But it will not create not any change. No mechanical advantage is observed.
 1
1 Answer:
52.6 gramStep-by-step explanation:
It is clear by the equation 2(27+3×35.5)= 267 gm of AlCl3 reacts with 6× 80 = 480 gm of Br2 . So 29.2 gm reacts = 480× 29.2/267= 52.6 gm
glycoproteins
Explanation:
A positive reaction for Molisch's test is given by almost all carbohydrates (exceptions include tetroses & trioses). It can be noted that even some glycoproteins and nucleic acids give positive results for this test (since they tend to undergo hydrolysis when exposed to strong mineral acids and form monosaccharides).
Answer:
Taking into accoun the ideal gas law, The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.
An ideal gas is a theoretical gas that is considered to be composed of point particles that move randomly and do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas. The universal constant of ideal gases R has the same value for all gaseous substances.
Explanation:
In this case, you know:
P= 0.884 atm
V= ?
n= 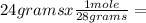 0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
R=0.082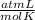
T= 328 K
Replacing in the ideal gas law:
0.884 atm×V= 0.857 moles× 0.082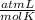 ×328 K
×328 K
Solving:
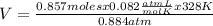
V= 26.07 L
The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.

It will provide an instant answer!
