 76
76 Answer
Answer 1 : 28.9 g of CO is needed.
Answer 2 : Six moles of  over Nine moles of
over Nine moles of 
Answer 3 : Four over two fraction can be used for the mole ratio to determine the mass of Fe from a known mass of  .
.
Answer 4 : Mass of  = (150 × 3 × 31.998) ÷ (232.29 × 1) grams
= (150 × 3 × 31.998) ÷ (232.29 × 1) grams
Answer 5 : 8.4 moles of sodium cyanide (NaCN) would be needed.
Solution
Solution 1 : Given,
Given mass of  = 55 g
= 55 g
Molar mass of  = 159.69 g/mole
= 159.69 g/mole
Molar mass of CO = 28.01 g/mole
Moles of  =
=  =
=  = 0.344 moles
= 0.344 moles
Balanced chemical reaction is,
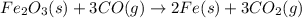
From the given reaction, we conclude that
1 mole of  gives → 3 moles of CO
gives → 3 moles of CO
0.344 moles of  gives → 3 × 0.344 moles of CO
gives → 3 × 0.344 moles of CO
= 1.032 moles
Mass of CO = Number of moles of CO × Molar mass of CO
= 1.032 × 28.01
= 28.90 g
Solution 2 : The balanced chemical reaction is,

From the given reaction, we conclude that the Six moles of  over Nine moles of
over Nine moles of  is the correct option.
is the correct option.
Solution 3 : The balanced chemical reaction is,

From the given balanced reaction, we conclude that Four over two fraction can be used for the mole ratio to determine the mass of Fe from a known mass of  .
.
Solution 4 : Given,
Given mass of  = 150 g
= 150 g
Molar mass of  = 232.29 g/mole
= 232.29 g/mole
Molar mass of  = 31.998 g/mole
= 31.998 g/mole
Moles of  =
=  =
= 
The balanced chemical equation is,

From the given balanced equation, we conclude that
1 mole of  gives → 3 moles of
gives → 3 moles of 
 of
of  gives →
gives → ![[(\frac{150\times 1}{232.29})\times 3] moles](/tpl/images/0390/3270/cd451.png) of
of 
Mass of  = Number of moles of
= Number of moles of  × Molar mass of
× Molar mass of  =
= ![[(\frac{150\times 1}{232.29})\times 3] \times 31.998 grams](/tpl/images/0390/3270/9e58a.png)
Therefore, the mass of  = (150 × 3 × 31.998) ÷ (232.29 × 1) grams
= (150 × 3 × 31.998) ÷ (232.29 × 1) grams
Solution 5 : Given,
Number of moles of  = 4.2 moles
= 4.2 moles
Balanced chemical equation is,

From the given chemical reaction, we conclude that
1 mole of  obtained from 2 moles of NaCN
obtained from 2 moles of NaCN
4.2 moles of  obtained → 2 × 4.2 moles of NaCN
obtained → 2 × 4.2 moles of NaCN
Therefore,
The moles of NaCN needed = 2 × 4.2 = 8.4 moles
 76
76 Answer
Answer 1 : 28.9 g of CO is needed.
Answer 2 : Six moles of  over Nine moles of
over Nine moles of 
Answer 3 : Four over two fraction can be used for the mole ratio to determine the mass of Fe from a known mass of  .
.
Answer 4 : Mass of  = (150 × 3 × 31.998) ÷ (232.29 × 1) grams
= (150 × 3 × 31.998) ÷ (232.29 × 1) grams
Answer 5 : 8.4 moles of sodium cyanide (NaCN) would be needed.
Solution
Solution 1 : Given,
Given mass of  = 55 g
= 55 g
Molar mass of  = 159.69 g/mole
= 159.69 g/mole
Molar mass of CO = 28.01 g/mole
Moles of  =
=  =
=  = 0.344 moles
= 0.344 moles
Balanced chemical reaction is,
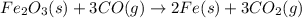
From the given reaction, we conclude that
1 mole of  gives → 3 moles of CO
gives → 3 moles of CO
0.344 moles of  gives → 3 × 0.344 moles of CO
gives → 3 × 0.344 moles of CO
= 1.032 moles
Mass of CO = Number of moles of CO × Molar mass of CO
= 1.032 × 28.01
= 28.90 g
Solution 2 : The balanced chemical reaction is,

From the given reaction, we conclude that the Six moles of  over Nine moles of
over Nine moles of  is the correct option.
is the correct option.
Solution 3 : The balanced chemical reaction is,

From the given balanced reaction, we conclude that Four over two fraction can be used for the mole ratio to determine the mass of Fe from a known mass of  .
.
Solution 4 : Given,
Given mass of  = 150 g
= 150 g
Molar mass of  = 232.29 g/mole
= 232.29 g/mole
Molar mass of  = 31.998 g/mole
= 31.998 g/mole
Moles of  =
=  =
= 
The balanced chemical equation is,

From the given balanced equation, we conclude that
1 mole of  gives → 3 moles of
gives → 3 moles of 
 of
of  gives →
gives → ![[(\frac{150\times 1}{232.29})\times 3] moles](/tpl/images/0390/3270/cd451.png) of
of 
Mass of  = Number of moles of
= Number of moles of  × Molar mass of
× Molar mass of  =
= ![[(\frac{150\times 1}{232.29})\times 3] \times 31.998 grams](/tpl/images/0390/3270/9e58a.png)
Therefore, the mass of  = (150 × 3 × 31.998) ÷ (232.29 × 1) grams
= (150 × 3 × 31.998) ÷ (232.29 × 1) grams
Solution 5 : Given,
Number of moles of  = 4.2 moles
= 4.2 moles
Balanced chemical equation is,

From the given chemical reaction, we conclude that
1 mole of  obtained from 2 moles of NaCN
obtained from 2 moles of NaCN
4.2 moles of  obtained → 2 × 4.2 moles of NaCN
obtained → 2 × 4.2 moles of NaCN
Therefore,
The moles of NaCN needed = 2 × 4.2 = 8.4 moles
 4
4  4
4 1. 10 moles of NO
2. 25 moles of NaCl
3. 1200 moles CO₂
4. 1.042 mole of MgO
5. 0.714 moles H₂ gas
6. 1040 g of BaCl₂
7. 9.5 g
8. 45.44 g of Au
9. 15 g of AlCl₃
Explanation:
Ans 1.
Data Given:
moles of Oxygen = 5 moles
moles of nitrogen monoxide = ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
N₂ + O₂ -----------> 2NO
1 mol 2 mol
So if we look at the reaction 1 mole Oxygen (O₂) gives 2 moles of nitrogen monoxide NO, then how many moles of nitrogen monoxide will be produced by 5 moles of Oxygen (O₂)
For this apply unity formula
1 mole of O₂ ≅ 2 moles of NO
5 mole of O₂ ≅ X moles of NO
By Doing cross multiplication
moles of NO = 2 moles x 5 moles / 1 mole
moles of NO = 10 mole
5 mole of O₂ will produce 10 moles of NO
________________
Ans 2.
Data Given:
moles of HCl = 25 moles
moles of NaCl = ?
Solution:
To solve this problem we have to look at the reaction
Neutralization Reaction:
HCl + NaOH -----------> NaCl + H₂O
1 mol 1 mol
So if we look at the reaction 1 mole HCl gives 1 moles of NaCl, then how many moles of NaCl will be formed by 25 moles of HCl
For this apply unity formula
1 mole of HCl ≅ 1 moles of NaCl
25 mole of HCl ≅ X moles of NaCl
By Doing cross multiplication
moles of NaCl = 1 moles x 25 moles / 1 mole
moles of NaCl = 25 mole
25 mole of HCL will form 25 moles of NaCl
________________
Ans 3.
Data Given:
moles of C₈H₁₈ = 150 moles
moles of CO₂= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
2C₈H₁₈ + 25O₂ -----------> 16CO₂ + 18H₂O
2 mol 16 mol
So if we look at the reaction 2 mole C₈H₁₈ gives 16 moles of CO₂, then how many moles of CO₂ will be Produce by 150 moles of C₈H₁₈
For this apply unity formula
2 mole of C₈H₁₈ ≅ 16 moles of CO₂
150 mole of C₈H₁₈ ≅ X moles of CO₂
By Doing cross multiplication
moles of CO₂= 16 moles x 150 moles / 2 mole
moles of CO₂ = 1200 mole
150 mole of C₈H₁₈ will form 1200 moles of CO₂
______________________
Ans 4.
Data Given:
mass of Mg = 25 g
moles of MgO= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
2Mg + O₂ -----------> 2MgO
2 mol 2 mol
Convert moles of Mg to mass
Molar mass of Mg = 24 g/mol
So,
2Mg + O₂ -----------> 2MgO
2 mol (24 g/mol) 2 mol
48 g 2 mol
So if we look at the reaction 48 g of Mg gives 2 moles of MgO, then how many moles of MgO will be Produce by 25 g of Mg
For this apply unity formula
48 g of Mg ≅ 2 moles of MgO
25 g of Mg ≅ X moles of MgO
By Doing cross multiplication
moles of MgO = 2 moles x 25 g / 48 g
moles of MgO = 1.042 mole
25 g of Mg will form 1.042 moles of MgO
______________________
Ans 5.
Data Given:
mass of Li = 10 g
moles of H₂= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
2Li + 2 H₂O -----------> 2LiOH + H₂
2 mol 1 mol
Convert moles of Li to mass
Molar mass of Li = 7 g/mol
So,
2Li + 2H₂O -----------> 2LiOH + H₂
2 mol (7 g/mol) 1 mol
14 g 1 mol
So if we look at the reaction 14 g of Li gives 1 moles of H₂ gas, then how many moles of H₂ gas will be Produce by 10 g of Li
For this apply unity formula
14 g of Li ≅ 1 moles of H₂
10 g of Li ≅ X moles of H₂
By Doing cross multiplication
moles of H₂ = 1 moles x 10 g / 14 g
moles of H₂ = 0.714 mole
10 g of Li will form 0.714 moles of H₂
______________________
Ans 6.
Data Given:
moles of Na₂SO₄= 5 moles
mass of BaCl₂ = ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
Na₂SO₄ + BaCl₂ -----------> 2NaCl + BaSO₄
1 mol 1 mol
Convert moles of BaCl₂ to mass
Molar mass of BaCl₂ = 208 g/mol
So,
Na₂SO₄ + BaCl₂ -----------> 2NaCl + BaSO₄
1 mol 1 mol (208 g/mol)
1 mol 208 g
So if we look at the reaction 1 mole of Na₂SO₄ react with 208 g of BaCl₂, Then how many grams of BaCl₂ will react with 5 moles of Na₂SO₄
For this apply unity formula
1 mole of Na₂SO₄ ≅ 208 g of BaCl₂
5 mole of Na₂SO₄≅ X g of BaCl₂
By Doing cross multiplication
mass of BaCl₂ = 208 g x 5 moles / 1 mole
mass of BaCl₂ = 1040 g
5 moles of Na₂SO₄ will react with 1040 g of BaCl₂
______________________
Ans 7.
Data Given:
mass of MgCO₃ = 20 g
moles of MgO= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
MgCO₃ -----------> MgO + CO₂
1 mol 1 mol
Convert moles to mass
Molar mass of MgCO₃ = 84 g/mol
Molar mass of MgO = 40 g/mol
So,
MgCO₃ -------------> MgO + CO₂
1 mol (84 g/mol) 1 mol (40 g/mol)
84 g 40 g
So if we look at the reaction 84 g of MgCO₃ gives 40 g of MgO, then how many g of MgO will be Produce by 20 g of MgCO₃
For this apply unity formula
84 g of MgCO₃ ≅ 40 g of MgO
20 g of MgCO₃ ≅ X g of MgO
By Doing cross multiplication
mass of MgO = 40 g x 20 g / 84g
mass of MgO = 9.5 g
20 g of MgCO₃ will produce 9.5 g of MgO
________________
The remaining portion is in attachment


1. 10 moles of NO
2. 25 moles of NaCl
3. 1200 moles CO₂
4. 1.042 mole of MgO
5. 0.714 moles H₂ gas
6. 1040 g of BaCl₂
7. 9.5 g
8. 45.44 g of Au
9. 15 g of AlCl₃
Explanation:
Ans 1.
Data Given:
moles of Oxygen = 5 moles
moles of nitrogen monoxide = ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
N₂ + O₂ -----------> 2NO
1 mol 2 mol
So if we look at the reaction 1 mole Oxygen (O₂) gives 2 moles of nitrogen monoxide NO, then how many moles of nitrogen monoxide will be produced by 5 moles of Oxygen (O₂)
For this apply unity formula
1 mole of O₂ ≅ 2 moles of NO
5 mole of O₂ ≅ X moles of NO
By Doing cross multiplication
moles of NO = 2 moles x 5 moles / 1 mole
moles of NO = 10 mole
5 mole of O₂ will produce 10 moles of NO
________________
Ans 2.
Data Given:
moles of HCl = 25 moles
moles of NaCl = ?
Solution:
To solve this problem we have to look at the reaction
Neutralization Reaction:
HCl + NaOH -----------> NaCl + H₂O
1 mol 1 mol
So if we look at the reaction 1 mole HCl gives 1 moles of NaCl, then how many moles of NaCl will be formed by 25 moles of HCl
For this apply unity formula
1 mole of HCl ≅ 1 moles of NaCl
25 mole of HCl ≅ X moles of NaCl
By Doing cross multiplication
moles of NaCl = 1 moles x 25 moles / 1 mole
moles of NaCl = 25 mole
25 mole of HCL will form 25 moles of NaCl
________________
Ans 3.
Data Given:
moles of C₈H₁₈ = 150 moles
moles of CO₂= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
2C₈H₁₈ + 25O₂ -----------> 16CO₂ + 18H₂O
2 mol 16 mol
So if we look at the reaction 2 mole C₈H₁₈ gives 16 moles of CO₂, then how many moles of CO₂ will be Produce by 150 moles of C₈H₁₈
For this apply unity formula
2 mole of C₈H₁₈ ≅ 16 moles of CO₂
150 mole of C₈H₁₈ ≅ X moles of CO₂
By Doing cross multiplication
moles of CO₂= 16 moles x 150 moles / 2 mole
moles of CO₂ = 1200 mole
150 mole of C₈H₁₈ will form 1200 moles of CO₂
______________________
Ans 4.
Data Given:
mass of Mg = 25 g
moles of MgO= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
2Mg + O₂ -----------> 2MgO
2 mol 2 mol
Convert moles of Mg to mass
Molar mass of Mg = 24 g/mol
So,
2Mg + O₂ -----------> 2MgO
2 mol (24 g/mol) 2 mol
48 g 2 mol
So if we look at the reaction 48 g of Mg gives 2 moles of MgO, then how many moles of MgO will be Produce by 25 g of Mg
For this apply unity formula
48 g of Mg ≅ 2 moles of MgO
25 g of Mg ≅ X moles of MgO
By Doing cross multiplication
moles of MgO = 2 moles x 25 g / 48 g
moles of MgO = 1.042 mole
25 g of Mg will form 1.042 moles of MgO
______________________
Ans 5.
Data Given:
mass of Li = 10 g
moles of H₂= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
2Li + 2 H₂O -----------> 2LiOH + H₂
2 mol 1 mol
Convert moles of Li to mass
Molar mass of Li = 7 g/mol
So,
2Li + 2H₂O -----------> 2LiOH + H₂
2 mol (7 g/mol) 1 mol
14 g 1 mol
So if we look at the reaction 14 g of Li gives 1 moles of H₂ gas, then how many moles of H₂ gas will be Produce by 10 g of Li
For this apply unity formula
14 g of Li ≅ 1 moles of H₂
10 g of Li ≅ X moles of H₂
By Doing cross multiplication
moles of H₂ = 1 moles x 10 g / 14 g
moles of H₂ = 0.714 mole
10 g of Li will form 0.714 moles of H₂
______________________
Ans 6.
Data Given:
moles of Na₂SO₄= 5 moles
mass of BaCl₂ = ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
Na₂SO₄ + BaCl₂ -----------> 2NaCl + BaSO₄
1 mol 1 mol
Convert moles of BaCl₂ to mass
Molar mass of BaCl₂ = 208 g/mol
So,
Na₂SO₄ + BaCl₂ -----------> 2NaCl + BaSO₄
1 mol 1 mol (208 g/mol)
1 mol 208 g
So if we look at the reaction 1 mole of Na₂SO₄ react with 208 g of BaCl₂, Then how many grams of BaCl₂ will react with 5 moles of Na₂SO₄
For this apply unity formula
1 mole of Na₂SO₄ ≅ 208 g of BaCl₂
5 mole of Na₂SO₄≅ X g of BaCl₂
By Doing cross multiplication
mass of BaCl₂ = 208 g x 5 moles / 1 mole
mass of BaCl₂ = 1040 g
5 moles of Na₂SO₄ will react with 1040 g of BaCl₂
______________________
Ans 7.
Data Given:
mass of MgCO₃ = 20 g
moles of MgO= ?
Solution:
To solve this problem we have to look at the reaction
Reaction:
MgCO₃ -----------> MgO + CO₂
1 mol 1 mol
Convert moles to mass
Molar mass of MgCO₃ = 84 g/mol
Molar mass of MgO = 40 g/mol
So,
MgCO₃ -------------> MgO + CO₂
1 mol (84 g/mol) 1 mol (40 g/mol)
84 g 40 g
So if we look at the reaction 84 g of MgCO₃ gives 40 g of MgO, then how many g of MgO will be Produce by 20 g of MgCO₃
For this apply unity formula
84 g of MgCO₃ ≅ 40 g of MgO
20 g of MgCO₃ ≅ X g of MgO
By Doing cross multiplication
mass of MgO = 40 g x 20 g / 84g
mass of MgO = 9.5 g
20 g of MgCO₃ will produce 9.5 g of MgO
________________
The remaining portion is in attachment


 10
10  10
10  2
2 1) 1.964 moles of O2
2) 0.670 moles of H2SO4
3) 2284.8 liters of oxygen
4) 24.3 grams of S
5) 1864.6 L of hydrogen gas
6) 19.54 grams of HCl
7) 45.0 L of CO2 produced
8) 36.0 L of NO
9) 30L of CO2
Explanation:
1. If 22 L of methane (CH4) gas burns (combustion), how many moles of oxygen will be needed for complete combustion?
Step 1: The balanced equation:
CH4 + 2O2 → CO2 + 2H2O
Step 2: Calculate moles of CH4
Since 22.4 L = 1.0 mol
22.4L = 0.982 moles of CH4
Step 3: Calculate moles of O2 needed
For 1 mol CH4 we need 2 moles of O2 to produce 1 mol of CO2 and 2 moles of H2O
For 0.982 moles of CH4 we need 2*0.982 = 1.964 moles of O2
2. Acid rain is produced when sulfur trioxide reacts with water in a composition reaction. If 15 L of sulfur trioxide is present in the air how many moles of sulfuric acid are produced?
Step 1: The balanced equation
SO3(g) + H2O(l) → H2SO4(aq)
Step 2: Calculate moles of SO3
22.4 L = 1 mol
15.0 L = 0.670 moles
Step 3: Calculate moles of H2SO4
For 1 mol of SO3 we need 1 mol of H2O to produce 1 mol of H2SO4
For 0.670 moles of SO3 we will produce 0.670 moles of H2SO4
3. If calcium chlorate is heated it decomposes into calcium chloride and oxygen gas.
How many liters of oxygen can be produced form heating 34 moles of calcium carbonate?
Step 1: The balanced equation
Ca(ClO3)2 → CaCl2 + 3O2
Step 2: Calculate moles of O2
For 1 mol of Ca(ClO3)2 consumed, we produce 1 mol of CaCl2 and 3 moles of O2
For 34 moles of Ca(ClO3)2 consumed, we'll have 3*34 = 102 moles of O2
Step 3: Calculate volume of oxygen
1 mol = 22.4 L
102 moles = 22.4 * 102 = 2284.8 liters of oxygen
4. Sulfur and oxygen combine to form sulfur dioxide in a composition reaction. How many grams of sulfur are required to react with 17 L of oxygen?
Step 1: The balanced equation
S + O2 → SO2
Step 2: Calculate number of moles of O2
22.4 L = 1 mol
17.0 L = 0.759 moles of O2
Step 3: Calculate moles of S
For 1 mol of S we need 1 mol of O2 to produce 1 mol of SO2
For 0.759 moles of O2 we need 0.759 moles of S
Step 4: Calculate mass of S
Mass of S = moles S * molar mass S
Mass of S = 0.759 moles * 32.065 g/mol = 24.3 grams of S
5. By running a direct current through water, it can be decomposed it into its elements. How many liters of hydrogen gas can be produced from 1500 g of water?
Step 1: The balanced equation
2H2O → 2H2 + O2
Step 2: Calculate number of moles of water
Moles H2O = mass H2O / molar mass H2O
Moles H2O = 1500 grams / 18.02 g/mol = 83.24 moles
Step 3: Calculate moles of H2
For 2 moles of H2O we'll have 2 moles of H2 and 1 mol of O2
For 83.24 moles of H2O we'll have 83.24 moles of H2
Step 4: Calculate volume of H2
1 mol = 22.4 L
83.24 moles = 22.4 * 83.24 = 1864.6 L of hydrogen gas
6. Hydrogen and chlorine can be combined to form hydrochloric acid. If you reacted 6 liters of hydrogen with chlorine how many grams of acid will be produced?
Step 1: The balanced equation
H2 + Cl2 → 2HCl
Step 2: Calculate moles of H2
22.4 L = 1 mol
6.0 L = 0.268 moles of H2 (Suppose H2 is the limiting reactant)
Step 3: Calculate moles of HCl
For 1 mol of H2 we need 1 mol of Cl2 to produce 2 moles of HCl
For 0.268 moles of H2 we'll have 2*0.268 = 0.536 moles of HCl
Step 4: Calculate mass of HCl
Mass HCl = moles HCl * molar mass HCl
Mass HCl = 0.536 * 36.46 g/mol
Mass HCl = 19.54 grams of HCl
7. . When glucose (C6H12O6) undergoes combustion with oxygen it produces water and carbon dioxide. How many liters of carbon dioxide will be produced if glucose reacts with 45 liters of oxygen?
Step 1: The balanced equation
C6H12O6 + 6O2 → 6CO2 + 6H2O
Step 2: Calculate moles of oxygen
22.4 L = 1 mol
45.0 L = 2.01 moles O2
Step 3: Calculate moles of CO2
For 1 mol of glucose we need 6 moles of O2 to prodcue 6 moles of CO2 and 6 moles of H2O
For 2.01 moles of O2 consumed we'll have 2.01 moles of CO2 produced
Step 4: Calculate liters of CO2 produced
1 mol = 22.4 L
2.01 moles = 45.0 L of CO2 produced
8. How many liters of nitrogen monoxide can be made using 18 liters of oxygen in the following composition reaction?
Step 1: The balanced equation:
N2 + O2 → 2NO
Step 2: Calculate number of moles of O2
22.4 L = 1.0 mol
18.0 L = 0.804 moles O2
Step 3: Calculate moles of NO
For 1 mol of N2 we need 1 mol of O2 to produce 2 moles of NO
For 0.804 moles of O2 we need 2*0.804 = 1.608 moles of NO
Step 4: Calculate volume of NO
1 mol = 22.4 L
1.608 moles = 22.4 * 1.608 = 36.0 L of NO
9. How many liters of carbon dioxide are produced when 10 liters of propane (C3H8) undergoes complete combustion? (assume STP)
Step 1: The balanced equation
C3H8 + 5O2 → 3CO2 + 4H2O
Step 2: Calculate moles of propane
22.4 L = 1.0 mol
10.0 L = 0.446 moles of propane
Step 3: Calculate moles of CO2
1 mol of propane consumed needs 5 moles of O2 to produce 3 moles of CO2 and 4 moles of H2O
For 0.446 moles of propane we'll have 3*0.446 = 1.338 moles of CO2
Step 4: Calculate volume of CO2
1 mol = 22.4 L
1.338 moles = 22.4 *1.338 = 30L of CO2
 2
2 1. D (24.0 moles CO2)
2. A (.239 moles H2)
Explanation:
1. First Balance the equation
1 C3H8 + 5 O2 ---> 3 CO2 + 4 H2O
Then set up a stoiciometric equation so that the moles of O2 cancel out
40mol O2 x 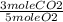 = 24.0 moles CO2
= 24.0 moles CO2
2. Set up a stoichiometric equation
10 grams Fe x 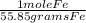 x
x 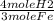 = 0.239 moles H2
= 0.239 moles H2

It will provide an instant answer!
