 11
11 The correct answers are:
17- C
18- C
19- B
20- C
Explanation:
QUESTION 17:
Both passages provide a vivid and extensive description of the core of the Earth. The first excerpt describes it from a subjective point of view; it is filled with adjectives qualifying the beauty and splendor of the elements found. The second one uses a scientific point of view, being completely objective in its definitions.
QUESTION 18:
The excerpt is used to show human's desire to learn and discover a great variety of new things. In this case, the two characters develop a gadget that can penetrate the core of the Earth only to satisfy their curiosity over what can be found there.
QUESTION 19:
While David quickly falls into despair and believes they have died and gone to heaven, Perry takes a moment to think and understand the situation. After a moment of silent he reaches the conclusion that they must be inside the Earth instead of on it. This shows the insight wisdom that comes with age contrasted with the impulsiveness of youth.
QUESTION 20:
This excerpt shows the fascination human beings feel for nature. In his description, David refers to the scene as both "weird and beautiful." He feels overwhelmed with the different species of trees and the glowing colors.
 11
11 The correct answers are:
17- C
18- C
19- B
20- C
Explanation:
QUESTION 17:
Both passages provide a vivid and extensive description of the core of the Earth. The first excerpt describes it from a subjective point of view; it is filled with adjectives qualifying the beauty and splendor of the elements found. The second one uses a scientific point of view, being completely objective in its definitions.
QUESTION 18:
The excerpt is used to show human's desire to learn and discover a great variety of new things. In this case, the two characters develop a gadget that can penetrate the core of the Earth only to satisfy their curiosity over what can be found there.
QUESTION 19:
While David quickly falls into despair and believes they have died and gone to heaven, Perry takes a moment to think and understand the situation. After a moment of silent he reaches the conclusion that they must be inside the Earth instead of on it. This shows the insight wisdom that comes with age contrasted with the impulsiveness of youth.
QUESTION 20:
This excerpt shows the fascination human beings feel for nature. In his description, David refers to the scene as both "weird and beautiful." He feels overwhelmed with the different species of trees and the glowing colors.
Answer:
AStep-by-step explanation:
The input force is 50 N. But it will not create not any change. No mechanical advantage is observed.
 1
1 Answer:
52.6 gramStep-by-step explanation:
It is clear by the equation 2(27+3×35.5)= 267 gm of AlCl3 reacts with 6× 80 = 480 gm of Br2 . So 29.2 gm reacts = 480× 29.2/267= 52.6 gm
Calcium (Ca)(On the periodic table, ionization energy increases as you go up and to the right of the periodic table)
glycoproteins
Explanation:
A positive reaction for Molisch's test is given by almost all carbohydrates (exceptions include tetroses & trioses). It can be noted that even some glycoproteins and nucleic acids give positive results for this test (since they tend to undergo hydrolysis when exposed to strong mineral acids and form monosaccharides).
Answer:
Taking into accoun the ideal gas law, The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.
An ideal gas is a theoretical gas that is considered to be composed of point particles that move randomly and do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas. The universal constant of ideal gases R has the same value for all gaseous substances.
Explanation:
In this case, you know:
P= 0.884 atm
V= ?
n= 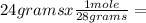 0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
R=0.082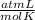
T= 328 K
Replacing in the ideal gas law:
0.884 atm×V= 0.857 moles× 0.082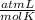 ×328 K
×328 K
Solving:
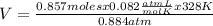
V= 26.07 L
The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.

It will provide an instant answer!
