 4
4  4
4 Explanation:
Given:
Area, A = 5 × 10^-6 m^2
Current, I = 30 A
U = (m × σ × V)/ρ × e × f × l)
= I/(n × A × Q)
Where,
u is again the drift velocity of the electrons, in m⋅s−1
m is the molecular mass of the metal, in kg
σ is the electric conductivity of the medium at the temperature considered, in S/m.
ΔV is the voltage applied across the conductor, in V
ρ is the density (mass per unit volume) of the conductor, in kg⋅m−3
e is the elementary charge, in C
f is the number of free electrons per atom
ℓ is the length of the conductor, in m
A.
Molar mass of iron = 56 g/mol
Converting g to kg,
56 g/mol × 1 kg/1000 g
= 0.056 kg/mol
= approx. 0.056 kilograms in 1 mole of iron.
B.
Density, ρ = 7874 kg/m^3
Molar density = density, ρ/molar mass
= 7874/0.056
= 1.406 × 10^5 mol/m^3
C.
Avogadros constant, Na = 6.022 × 10^23 atoms/mol
Density of iron atoms = avogadros constant × molar density
= 6.022 × 10^23 × 1.406 × 10^5
= 8.478 × 10^28 atoms/m^3
D.
Fe --> Fe2+ + 2e-
density of conduction electrons = 2 conduction electrons/1 atom of iron
= 2 × 8.478 × 10^28
= 1.69 × 10^29 conduction electrons/m^3
E.
Q = 1.602 × 10^-19 C
Using the equation above,
V = 30/(1.69 × 10^29 × 1.602 × 10^-19 × 5 × 10^-6)
= 2.216 × 10^-4 m/s.
Explanation:
Given:
Area, A = 5 × 10^-6 m^2
Current, I = 30 A
U = (m × σ × V)/ρ × e × f × l)
= I/(n × A × Q)
Where,
u is again the drift velocity of the electrons, in m⋅s−1
m is the molecular mass of the metal, in kg
σ is the electric conductivity of the medium at the temperature considered, in S/m.
ΔV is the voltage applied across the conductor, in V
ρ is the density (mass per unit volume) of the conductor, in kg⋅m−3
e is the elementary charge, in C
f is the number of free electrons per atom
ℓ is the length of the conductor, in m
A.
Molar mass of iron = 56 g/mol
Converting g to kg,
56 g/mol × 1 kg/1000 g
= 0.056 kg/mol
= approx. 0.056 kilograms in 1 mole of iron.
B.
Density, ρ = 7874 kg/m^3
Molar density = density, ρ/molar mass
= 7874/0.056
= 1.406 × 10^5 mol/m^3
C.
Avogadros constant, Na = 6.022 × 10^23 atoms/mol
Density of iron atoms = avogadros constant × molar density
= 6.022 × 10^23 × 1.406 × 10^5
= 8.478 × 10^28 atoms/m^3
D.
Fe --> Fe2+ + 2e-
density of conduction electrons = 2 conduction electrons/1 atom of iron
= 2 × 8.478 × 10^28
= 1.69 × 10^29 conduction electrons/m^3
E.
Q = 1.602 × 10^-19 C
Using the equation above,
V = 30/(1.69 × 10^29 × 1.602 × 10^-19 × 5 × 10^-6)
= 2.216 × 10^-4 m/s.
I cannot give you all the answer but I can help you to solve those.
Explanation:
The first question:
How many grams are there in 7.5×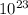 molecules of
molecules of 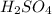 ?
?
So we need to find the molecular mass first, use your periodic table,
And then we can find out 2+32+16×4=98 g/mol
Then, we need to find how many moles, by using Avogadro's constant:
Avogadro's constant: 1 mole = 6.02×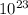
∴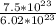 =1.25 mol(2d.p.)
=1.25 mol(2d.p.)
Lastly, find the grams using the formula 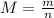
m=Mn
m=1.25*98
m=122.5g
-------------------------------------------------------------------------------------------------------------
In conclusion, use those formula to help you:
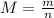 (which M = molecular mass(atomic mass) m=mass of the substance and n = moles)
(which M = molecular mass(atomic mass) m=mass of the substance and n = moles)
Avogadro's constant: 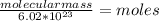
I cannot give you all the answer but I can help you to solve those.
Explanation:
The first question:
How many grams are there in 7.5×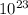 molecules of
molecules of 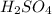 ?
?
So we need to find the molecular mass first, use your periodic table,
And then we can find out 2+32+16×4=98 g/mol
Then, we need to find how many moles, by using Avogadro's constant:
Avogadro's constant: 1 mole = 6.02×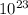
∴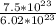 =1.25 mol(2d.p.)
=1.25 mol(2d.p.)
Lastly, find the grams using the formula 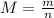
m=Mn
m=1.25*98
m=122.5g
-------------------------------------------------------------------------------------------------------------
In conclusion, use those formula to help you:
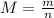 (which M = molecular mass(atomic mass) m=mass of the substance and n = moles)
(which M = molecular mass(atomic mass) m=mass of the substance and n = moles)
Avogadro's constant: 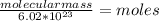
 2
2 In 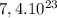 molecules of
molecules of 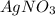 are 209 g
are 209 g
In 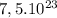 molecules of
molecules of 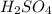 are 123 g
are 123 g
In 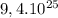 molecules of
molecules of 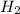 are 312 g
are 312 g
Explanation:
First remember that 1 mole of any element has 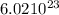 molecules (this number is a constant).
molecules (this number is a constant).
we know that the molecular mass of an element relates the grams of that substance in 1 mol
To calculate how many grams of 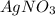 are in
are in 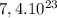 molecules we use a rule of three
molecules we use a rule of three
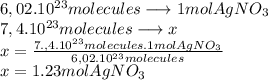
The molecular mass of 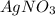 is 169.87 g/mol
is 169.87 g/mol
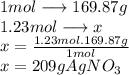
In  molecules of
molecules of 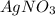 are 209 g
are 209 g
To calculate how many grams of 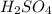 are in
are in 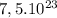 molecules we repeat the previous procedure
molecules we repeat the previous procedure
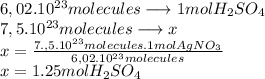
The molecular mass of 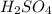 is 98.079 g/mol
is 98.079 g/mol
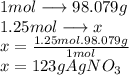
In 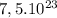 molecules of
molecules of 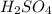 are 123 g
are 123 g
To calculate how many grams of 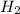 are in
are in 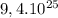 molecules we repeat the previous procedure
molecules we repeat the previous procedure
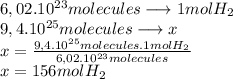
The molecular mass of 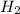 is 2 g/mol
is 2 g/mol
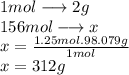
In 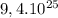 molecules of
molecules of 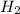 are 312 g
are 312 g
 2
2 In 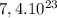 molecules of
molecules of 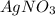 are 209 g
are 209 g
In 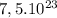 molecules of
molecules of 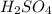 are 123 g
are 123 g
In 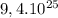 molecules of
molecules of 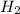 are 312 g
are 312 g
Explanation:
First remember that 1 mole of any element has 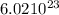 molecules (this number is a constant).
molecules (this number is a constant).
we know that the molecular mass of an element relates the grams of that substance in 1 mol
To calculate how many grams of 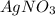 are in
are in 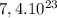 molecules we use a rule of three
molecules we use a rule of three
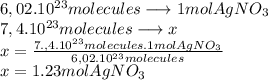
The molecular mass of 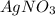 is 169.87 g/mol
is 169.87 g/mol
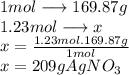
In 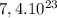 molecules of
molecules of 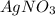 are 209 g
are 209 g
To calculate how many grams of 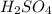 are in
are in 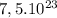 molecules we repeat the previous procedure
molecules we repeat the previous procedure
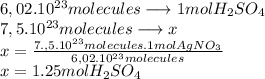
The molecular mass of 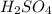 is 98.079 g/mol
is 98.079 g/mol
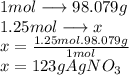
In 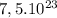 molecules of
molecules of 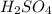 are 123 g
are 123 g
To calculate how many grams of 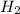 are in
are in 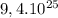 molecules we repeat the previous procedure
molecules we repeat the previous procedure
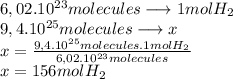
The molecular mass of 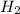 is 2 g/mol
is 2 g/mol
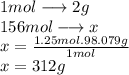
In 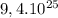 molecules of
molecules of 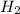 are 312 g
are 312 g

It will provide an instant answer!
