For 1: The correct answer is False.
For 2: The correct answer is True.
For 3: The correct answer is True.
For 4: The correct answer is False.
For 5: The correct answer is True.
Explanation:
Net ionic equation of any reaction does not include any spectator ions. If no net ionic equation is formed, it is said that no reaction has occurred.
Spectator ions are defined as the ions which does not get involved in a chemical equation. They are found on both the sides of the chemical reaction when it is present in ionic form. Solids, liquids and gases do not exist as ions.
For 1: Lead(II) nitrate and sodium chlorideThe chemical equation for the reaction of lead (II) nitrate and sodium chloride is given as:

Ionic form of the above equation follows:

As, sodium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:

Hence, the correct answer is False.
For 2: Sodium bromide and hydrochloric acidThe chemical equation for the reaction of sodium bromide and hydrochloric acid is given as:

Ionic form of the above equation follows:
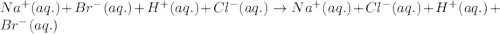
There are no spectator ions in the equation. So, the above reaction is the net ionic equation.
Hence, the correct answer is True.
For 3: Nickel (II) chloride and lead(II) nitrateThe chemical equation for the reaction of lead (II) nitrate and nickel (II) chloride is given as:

Ionic form of the above equation follows:

As, nickel and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:

Hence, the correct answer is True.
For 4: Magnesium chloride and sodium hydroxideThe chemical equation for the reaction of magnesium chloride and sodium hydroxide is given as:

Ionic form of the above equation follows:

As, sodium and chloride ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:
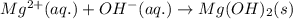
Hence, the correct answer is False.
For 5: Ammonium sulfate and barium nitrateThe chemical equation for the reaction of ammonium sulfate and barium nitrate is given as:

Ionic form of the above equation follows:

As, ammonium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:
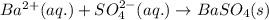
Hence, the correct answer is True.
For 1: The correct answer is False.
For 2: The correct answer is True.
For 3: The correct answer is True.
For 4: The correct answer is False.
For 5: The correct answer is True.
Explanation:
Net ionic equation of any reaction does not include any spectator ions. If no net ionic equation is formed, it is said that no reaction has occurred.
Spectator ions are defined as the ions which does not get involved in a chemical equation. They are found on both the sides of the chemical reaction when it is present in ionic form. Solids, liquids and gases do not exist as ions.
For 1: Lead(II) nitrate and sodium chlorideThe chemical equation for the reaction of lead (II) nitrate and sodium chloride is given as:

Ionic form of the above equation follows:

As, sodium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:

Hence, the correct answer is False.
For 2: Sodium bromide and hydrochloric acidThe chemical equation for the reaction of sodium bromide and hydrochloric acid is given as:

Ionic form of the above equation follows:
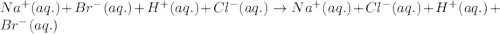
There are no spectator ions in the equation. So, the above reaction is the net ionic equation.
Hence, the correct answer is True.
For 3: Nickel (II) chloride and lead(II) nitrateThe chemical equation for the reaction of lead (II) nitrate and nickel (II) chloride is given as:

Ionic form of the above equation follows:

As, nickel and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:

Hence, the correct answer is True.
For 4: Magnesium chloride and sodium hydroxideThe chemical equation for the reaction of magnesium chloride and sodium hydroxide is given as:

Ionic form of the above equation follows:

As, sodium and chloride ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:
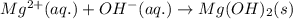
Hence, the correct answer is False.
For 5: Ammonium sulfate and barium nitrateThe chemical equation for the reaction of ammonium sulfate and barium nitrate is given as:

Ionic form of the above equation follows:

As, ammonium and nitrate ions are present on both the sides of the reaction. Thus, it will not be present in the net ionic equation and are spectator ions.
The net ionic equation for the above reaction follows:
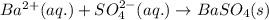
Hence, the correct answer is True.
A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
a) sodium hydroxide + ammonium sulfate :

b) niobium(V) sulfate + barium nitrate:

c) strontium bromide + silver nitrate:

A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
a) sodium hydroxide + ammonium sulfate :

b) niobium(V) sulfate + barium nitrate:

c) strontium bromide + silver nitrate:

 4
4  3
3  5
5  3
3  4
4  23
23 1.What changes occur to chemical bonds during a chemical reaction?
The chemical bonds will be altered or rearranged in chemical reaction. The bond that exist prior of the reaction could be separated or new bond will be formed. The rearrangement of the bond will change the properties of the molecule.
2.How does the change in energy of a chemical reaction predict whether or not the reaction will occur?
Chemical reaction happens when some subtrate molecule/atom collide. To start a chemical reaction there will be a certain amount of energy needed called activation energy. If the energy is not reaching activation energy, the reaction will not occur.
3.Explain the role of enzymes and how they affect the chemical reactions of living things.
Enzyme help chemical reaction by lowering the activation energy of the reaction, make the reaction faster and easier. Living things need to do some chemical reaction to produce and utilize energy. Enzyme could help those reaction, make the organism to produce and utilize energy faster. This will help the living things to fulfill their energy needs.
4.Explain why a key that fits into a lock is a useful model for the function of enzymes.
Key and lock model state that the enzyme is like a key that could only work with a certain type of lock. This because the enzyme has a specific active site in their three dimensional structure that could hold a substrate, and help the chemical reaction. Enzyme only work with substrate that could be hold by their active site, same as "lock" could only work with specific "key".
5.Explain how consuming an acid-neutralizing antacid might affect protein digestion.Apply the concept of activation energy to support your explanation.
Protein digestion happens in the stomach by pepsin enzyme. The ideal condition of the enzyme to work is in acid pH. Using antacid will cause the pH to become neutral. This will alter the enzyme structure, make the enzyme didn't work. If the enzyme didn't work, the activation energy will be much higher and protein digestion will be slower.
6.Predict which temperature (20degrees Celsius ,39 degrees celsius, or 50 degree Celsius) --- you would expect a human enzyme to function best.Plan a simple investigation to test your hypothesis.
Human is a warm blooded mammals that keep their body temperature around 37 degrees celsius. The ideal condition of human enzyme activity would be around that temperature. From the option, 39 degrees would be the most appropriate as it was the closest to 37 degrees.
You can investigate this by taking some enzyme and examine their speed in container with different temperature.

It will provide an instant answer!
