Answer:
AStep-by-step explanation:
The input force is 50 N. But it will not create not any change. No mechanical advantage is observed.
 1
1 Answer:
52.6 gramStep-by-step explanation:
It is clear by the equation 2(27+3×35.5)= 267 gm of AlCl3 reacts with 6× 80 = 480 gm of Br2 . So 29.2 gm reacts = 480× 29.2/267= 52.6 gm
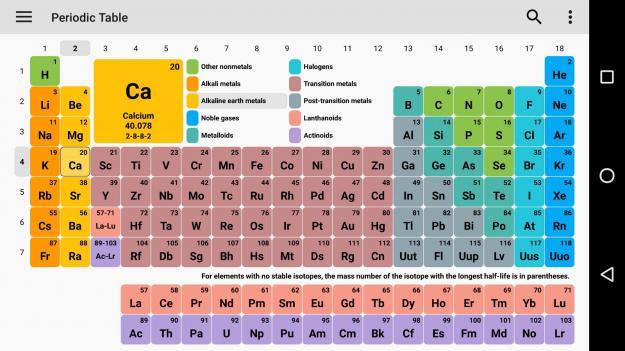
Calcium (Ca)(On the periodic table, ionization energy increases as you go up and to the right of the periodic table)
 1
1 
It will provide an instant answer!
