 2
2 Part A:
The balanced chemical equation for the reaction is
2C₈H₁₈(g) + 25O₂(g) → 16CO₂(g) + 18H₂O(g)
The coefficients for each compound in order is 2, 25, 16, 18
Part B:
The limiting reactant is Oxygen
Part C:
The number of mole of water produced is 0.454 mol
Part D:
The number of moles of octane left is 0.230 mol
Part AFor the reaction,
The balanced chemical equation for the reaction is
2C₈H₁₈(g) + 25O₂(g) → 16CO₂(g) + 18H₂O(g)
This means 2 moles of C₈H₁₈ will react with 25 moles of O₂ to produce 16 moles of CO₂ and 18 moles of H₂O
Hence, the coefficients for each compound in order is 2, 25, 16, 18
Part BIf 0.280 mol of octane is allowed to react with 0.630 mol of oxygen,
To determine the limiting reagent, we will calculate the number of moles of oxygen that is required to react with the 0.280 mol of octane
Since,
2 moles of octane reacts with 25 moles of oxygen,
Then,
0.280 mole of octane will react with  moles of oxygen
moles of oxygen
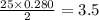
∴ 3.5 moles of oxygen is required to react with the 0.280 mole of octane.
Since the number of moles of oxygen present (0.630 mole) is less than the required amount of oxygen needed (3.5 mole), then, oxygen is the limiting reactant
Hence, the limiting reactant is Oxygen
Part CTo determine the number of moles of water produced,
First, we will calculate the number of moles of octane that reacted
From the balanced chemical equation
2 moles of octane reacts with 25 moles of oxygen
Then,
 moles of octane will react with the 0.630 mole of oxygen present
moles of octane will react with the 0.630 mole of oxygen present

∴ Only 0.0504 mole of octane reacted
Now,
If 2 moles of octane react with 25 moles of O₂ to produce 18 moles of water,
Then,
0.0504 mole of octane will react with 0.630 mole of oxygen to produce 0.0504 × 9 mole of water
0.0504 × 9 = 0.4536 mol ≅ 0.454 mol
Hence, the number of mole of water produced is 0.454 mol
Part DFor the quantity of octane that is left
Number of moles of octane left = Number of moles of octane present - Number of moles of octane that reacted
Number of moles of octane left = 0.280 mol - 0.0504 mol
Number of moles of octane left = 0.2296 mol
Number of moles of octane left ≅ 0.230 mol
Hence, the number of moles of octane left is 0.230 mol
Learn more here: link
 2
2 Part A:
The balanced chemical equation for the reaction is
2C₈H₁₈(g) + 25O₂(g) → 16CO₂(g) + 18H₂O(g)
The coefficients for each compound in order is 2, 25, 16, 18
Part B:
The limiting reactant is Oxygen
Part C:
The number of mole of water produced is 0.454 mol
Part D:
The number of moles of octane left is 0.230 mol
Part AFor the reaction,
The balanced chemical equation for the reaction is
2C₈H₁₈(g) + 25O₂(g) → 16CO₂(g) + 18H₂O(g)
This means 2 moles of C₈H₁₈ will react with 25 moles of O₂ to produce 16 moles of CO₂ and 18 moles of H₂O
Hence, the coefficients for each compound in order is 2, 25, 16, 18
Part BIf 0.280 mol of octane is allowed to react with 0.630 mol of oxygen,
To determine the limiting reagent, we will calculate the number of moles of oxygen that is required to react with the 0.280 mol of octane
Since,
2 moles of octane reacts with 25 moles of oxygen,
Then,
0.280 mole of octane will react with  moles of oxygen
moles of oxygen
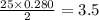
∴ 3.5 moles of oxygen is required to react with the 0.280 mole of octane.
Since the number of moles of oxygen present (0.630 mole) is less than the required amount of oxygen needed (3.5 mole), then, oxygen is the limiting reactant
Hence, the limiting reactant is Oxygen
Part CTo determine the number of moles of water produced,
First, we will calculate the number of moles of octane that reacted
From the balanced chemical equation
2 moles of octane reacts with 25 moles of oxygen
Then,
 moles of octane will react with the 0.630 mole of oxygen present
moles of octane will react with the 0.630 mole of oxygen present

∴ Only 0.0504 mole of octane reacted
Now,
If 2 moles of octane react with 25 moles of O₂ to produce 18 moles of water,
Then,
0.0504 mole of octane will react with 0.630 mole of oxygen to produce 0.0504 × 9 mole of water
0.0504 × 9 = 0.4536 mol ≅ 0.454 mol
Hence, the number of mole of water produced is 0.454 mol
Part DFor the quantity of octane that is left
Number of moles of octane left = Number of moles of octane present - Number of moles of octane that reacted
Number of moles of octane left = 0.280 mol - 0.0504 mol
Number of moles of octane left = 0.2296 mol
Number of moles of octane left ≅ 0.230 mol
Hence, the number of moles of octane left is 0.230 mol
Learn more here: link
 2
2 15L of 0.40M NaCl(aq) solution
Explanation:
2NaCl(aq) + 2H₂O(l) → 2NaOH(aq) + Cl₂(g)
2Na⁺(aq) + 2Clˉ(aq) + 2H₂O(l) → 2Na⁺(aq) + 2OHˉ(aq) + Cl₂(g)
Na⁺(aq) is a spectator ion in the given reaction and does not enter into the reaction process…
Net Ionic Equation is then 2Clˉ(aq) + 2H₂O(l) → 2OHˉ(aq) + Cl₂(g)
From Rxn, 2 moles Clˉ(aq) is needed to produce 1 mole of Cl₂(g)
Therefore, 6 moles Clˉ(aq) is needed to produce 3 moles of Cl₂(g)
That is, 6 moles NaCl(aq) → 6 moles Clˉ(aq) = 0.40M x V(NaCl)liters
V(NaCl) liters = 6 moles Clˉ(aq)/(0.40mole/liter) = 15 liters of 0.40M NaCl(aq)
 2
2 15L of 0.40M NaCl(aq) solution
Explanation:
2NaCl(aq) + 2H₂O(l) → 2NaOH(aq) + Cl₂(g)
2Na⁺(aq) + 2Clˉ(aq) + 2H₂O(l) → 2Na⁺(aq) + 2OHˉ(aq) + Cl₂(g)
Na⁺(aq) is a spectator ion in the given reaction and does not enter into the reaction process…
Net Ionic Equation is then 2Clˉ(aq) + 2H₂O(l) → 2OHˉ(aq) + Cl₂(g)
From Rxn, 2 moles Clˉ(aq) is needed to produce 1 mole of Cl₂(g)
Therefore, 6 moles Clˉ(aq) is needed to produce 3 moles of Cl₂(g)
That is, 6 moles NaCl(aq) → 6 moles Clˉ(aq) = 0.40M x V(NaCl)liters
V(NaCl) liters = 6 moles Clˉ(aq)/(0.40mole/liter) = 15 liters of 0.40M NaCl(aq)
 3
3 148 g H₂SO₄
General Formulas and Concepts:
Chemistry - Stoichiometry
Reading a Periodic TableBalancing RxN'sUsing Dimensional AnalysisExplanation:
Step 1: Define
RxN: Ca(OH)₂ + H₂SO₄ → CaSO₄ + H₂O
Given: 3.01 moles H₂O
Step 2: Balance RxN
Ca(OH)₂ + H₂SO₄ → CaSO₄ + 2H₂O
Need the same amount of O's and H's on both sidesStep 3: Define conversions
Molar Mass of H - 1.01 g/mol
Molar Mass of S - 32.07 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of H₂SO₄ - 2(1.01) + 32.07 + 4(16.00) = 98.09 g/mol
Step 4: Stoichiometry
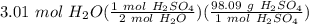 = 147.625 g H₂SO₄
= 147.625 g H₂SO₄
Step 5: Check
We are given 3 sig figs. Follow sig fig rules.
147.625 g H₂SO₄ ≈ 148 g H₂SO₄
 3
3 148 g H₂SO₄
General Formulas and Concepts:
Chemistry - Stoichiometry
Reading a Periodic TableBalancing RxN'sUsing Dimensional AnalysisExplanation:
Step 1: Define
RxN: Ca(OH)₂ + H₂SO₄ → CaSO₄ + H₂O
Given: 3.01 moles H₂O
Step 2: Balance RxN
Ca(OH)₂ + H₂SO₄ → CaSO₄ + 2H₂O
Need the same amount of O's and H's on both sidesStep 3: Define conversions
Molar Mass of H - 1.01 g/mol
Molar Mass of S - 32.07 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of H₂SO₄ - 2(1.01) + 32.07 + 4(16.00) = 98.09 g/mol
Step 4: Stoichiometry
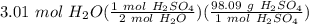 = 147.625 g H₂SO₄
= 147.625 g H₂SO₄
Step 5: Check
We are given 3 sig figs. Follow sig fig rules.
147.625 g H₂SO₄ ≈ 148 g H₂SO₄
Calcium (Ca)(On the periodic table, ionization energy increases as you go up and to the right of the periodic table)
glycoproteins
Explanation:
A positive reaction for Molisch's test is given by almost all carbohydrates (exceptions include tetroses & trioses). It can be noted that even some glycoproteins and nucleic acids give positive results for this test (since they tend to undergo hydrolysis when exposed to strong mineral acids and form monosaccharides).
Answer:
Taking into accoun the ideal gas law, The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.
An ideal gas is a theoretical gas that is considered to be composed of point particles that move randomly and do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas. The universal constant of ideal gases R has the same value for all gaseous substances.
Explanation:
In this case, you know:
P= 0.884 atm
V= ?
n= 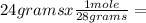 0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
R=0.082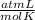
T= 328 K
Replacing in the ideal gas law:
0.884 atm×V= 0.857 moles× 0.082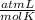 ×328 K
×328 K
Solving:
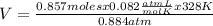
V= 26.07 L
The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.

It will provide an instant answer!
