The correct answers are (1) True (2) London dispersion force (3) one of the option is missing, none of these three is an answer (4) The total number of electrons increases down the group, and this increases the strength of the intermolecular forces (5) London dispersion forces which are always present.
Explanation:
Given: An instantaneous dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized.
True
when the electrons moving in a molecule or an atom move towards one end of the molecule or atom the other end has a small positive pole at that time and the end where electrons move has a small negative pole. So, a dipole is formed for that instant.
(2) What type of intermolecular force occurs between all substances?
London dispersion force
remember from first part how an instantaneous dipole is formed. When a pole is formed at that instant the molecule in neighborhood can interact with the dipole that was formed. The dipole that was formed has a positive pole and a negative pole. If the neighboring molecule is present near positive pole it’s electrons will get attracted to the positive pole of the dipole. This interaction is called London dispersion force. Since every atom or molecule or ion in the universe has electrons, so there is development of instantaneous dipole in each of them and each one of them has London dispersion force acting on them.
(3) When comparing 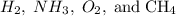 which of the following statements is correct?
which of the following statements is correct?
one of the option is missing, none of these three is an answer.
(4)The boiling points of diatomic halogens are compared in the table.
The total number of electrons increases down the group, and this increases the strength of the intermolecular forces.
(5) What is the strongest intermolecular force that occurs between molecules of 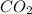 ?
?
London dispersion forces which are always present.
London forces are weakest forces, if other forces would have been present then we would have ignored London forces but since no other forces are present, the only choice is London forces.
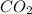 is a non polar molecule, it does not have permanent dipole moment. So there are no chances of dipole-dipole,
is a non polar molecule, it does not have permanent dipole moment. So there are no chances of dipole-dipole, 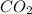 doesn’t have a positive or negative charge so even not ionic bond.
doesn’t have a positive or negative charge so even not ionic bond.
Learn more about dipole here:
link