 1
1 Do you have the answers i need help
 1
1 Do you have the answers i need help
 2
2 139.18 g.
Explanation:
For the balanced equation:CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l),
It is clear that 1 mol of CH₄ react with 2 mol of O₂ to produce 1 mol of CO₂ and 2 mol of H₂O.
Firstly, we need to calculate the no. of moles of 50.6 g of CH₄:no. of moles of CH₄ = mass/molar mass = (50.6 g)/(16.0 g/mol) = 3.1625 mol.
Using cross-multiplication:
1.0 mol of CH₄(g) produces → 1.0 mol of CO₂, from stichiometry.
∴ 3.1625 mol of CH₄(g) produces → 3.1625 mol of CO₂.
∴ The no. of grams of CO₂ produced = (no. of moles of CO₂)(molar mass of CO₂) = (3.1625 mol)(44.01 g/mol) = 139.18 g.
 2
2 139.18 g.
Explanation:
For the balanced equation:CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l),
It is clear that 1 mol of CH₄ react with 2 mol of O₂ to produce 1 mol of CO₂ and 2 mol of H₂O.
Firstly, we need to calculate the no. of moles of 50.6 g of CH₄:no. of moles of CH₄ = mass/molar mass = (50.6 g)/(16.0 g/mol) = 3.1625 mol.
Using cross-multiplication:
1.0 mol of CH₄(g) produces → 1.0 mol of CO₂, from stichiometry.
∴ 3.1625 mol of CH₄(g) produces → 3.1625 mol of CO₂.
∴ The no. of grams of CO₂ produced = (no. of moles of CO₂)(molar mass of CO₂) = (3.1625 mol)(44.01 g/mol) = 139.18 g.
HCl is the limiting reactant and the theoretical yield is 2.72 g of CO2. If the actual yield was 2.50 g then, the percent yield is 92.0% when rounding off is done only for the final answer.
Further Explanation:
In order to determine the theoretical yield and the percent yield of CO2, the following steps must be done:
Determine the limiting reactant. This is the reactant that will determine the amount of CO2 that will actually form. Determine the theoretical yield for CO2 when the limiting reactant is used.Get the percent yield by getting the ratio of the actual yield stated in the problem and the calculated theoretical yield multiplied by 100.Determining the Limiting Reactant
The Limiting Reactant (LR) will produce fewer moles of the products. To check which of the reactants HCl or CaCO3 is the LR, we do dimensional analysis:
For HCl:

For CaCO3:

Since HCl produces fewer moles of CO2, then it is the limiting reactant. We will use the given amount to determine the theoretical yield for CO2.
Determining the Theoretical Yield
From Step 1, we know that 0.0617098 moles of CO2 will be produced. We will just convert this to grams.
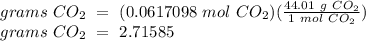
Since the answer only requires 3 significant figures, the final answer is 2.72 grams CO2.
Determining the Percent Yield
Dividing the actual yield by the theoretical yield will give us the percent yield, which is an indicator of how efficient the experiment or the method used was.
From the problem, the actual yield was 2.50 g, hence, the percent yield is:

Rounding off to three significant figures, the percent yield is 92.0%. This suggests that the method used is somewhat efficient in producing CO2.
Learn More
Learn More about Limiting Reactant link Learn More about Excess Reactant link Learn More about Stoichiometry linkKeywords: stoichiometry, theoretical yield, actual yield
 9
9 Explanation:
Pair A:
nitrogen
Moles of oxygen:
Number of moles = mass/ molar mass
Number of moles = 2.50 g/ 32 g/mol
Number of moles = 0.08 mol
Moles of nitrogen:
Number of moles = mass/ molar mass
Number of moles = 2.50 g/ 28 g/mol
Number of moles = 0.09 mol
Pair B:
nitrogen
Moles of nitrogen:
Number of moles = mass/ molar mass
Number of moles = 21.50 g/ 28 g/mol
Number of moles = 0.77 mol
Moles of carbon dioxide:
Number of moles = mass/ molar mass
Number of moles = 15.2 g/ 44 g/mol
Number of moles = 0.341 mol
Pair C:
Both have same number of moles.
Moles of oxygen:
Number of moles = mass/ molar mass
Number of moles = 0.054 g/ 32 g/mol
Number of moles = 0.002 mol
Moles of carbon dioxide:
Number of moles = mass/ molar mass
Number of moles = 0.081 g/ 44 g/mol
Number of moles = 0.002 mol
 ](V-nb) = nRT;
[P + 3.59 atm·L²mol⁻²
](V-nb) = nRT;
[P + 3.59 atm·L²mol⁻²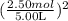 ](5.00 L - 2.50 mol × 0.0427 mol·L⁻¹) = 2.50 mol × 0.08206 L·atm·K⁻¹mol⁻¹ × 450 K;
(P + 0.898 atm)(5.00 L - 0.107 L) = 92.3 L·atm;
(P + 0.898 atm)(4.89) = 92.3 atm;
P + 0.898 atm =
](5.00 L - 2.50 mol × 0.0427 mol·L⁻¹) = 2.50 mol × 0.08206 L·atm·K⁻¹mol⁻¹ × 450 K;
(P + 0.898 atm)(5.00 L - 0.107 L) = 92.3 L·atm;
(P + 0.898 atm)(4.89) = 92.3 atm;
P + 0.898 atm = 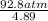 = 18.87 atm;
P = 18.87 atm - 0.898 atm = 17.97 atm
= 18.87 atm;
P = 18.87 atm - 0.898 atm = 17.97 atmHCl is the limiting reactant and the theoretical yield is 2.72 g of CO2. If the actual yield was 2.50 g then, the percent yield is 92.0% when rounding off is done only for the final answer.
Further Explanation:
In order to determine the theoretical yield and the percent yield of CO2, the following steps must be done:
Determine the limiting reactant. This is the reactant that will determine the amount of CO2 that will actually form. Determine the theoretical yield for CO2 when the limiting reactant is used.Get the percent yield by getting the ratio of the actual yield stated in the problem and the calculated theoretical yield multiplied by 100.Determining the Limiting Reactant
The Limiting Reactant (LR) will produce fewer moles of the products. To check which of the reactants HCl or CaCO3 is the LR, we do dimensional analysis:
For HCl:

For CaCO3:

Since HCl produces fewer moles of CO2, then it is the limiting reactant. We will use the given amount to determine the theoretical yield for CO2.
Determining the Theoretical Yield
From Step 1, we know that 0.0617098 moles of CO2 will be produced. We will just convert this to grams.
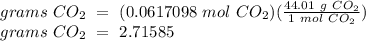
Since the answer only requires 3 significant figures, the final answer is 2.72 grams CO2.
Determining the Percent Yield
Dividing the actual yield by the theoretical yield will give us the percent yield, which is an indicator of how efficient the experiment or the method used was.
From the problem, the actual yield was 2.50 g, hence, the percent yield is:

Rounding off to three significant figures, the percent yield is 92.0%. This suggests that the method used is somewhat efficient in producing CO2.
Learn More
Learn More about Limiting Reactant link Learn More about Excess Reactant link Learn More about Stoichiometry linkKeywords: stoichiometry, theoretical yield, actual yield

It will provide an instant answer!
