 2
2 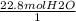 *
* 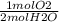 =
=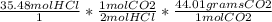 =
= 1
1 1. 4.57x10²³ atoms ; 2. 0.093 moles; 3. 4.5 grams ; 4. 0.434 moles , 5. 0.5 mol of Cl₂ ; 6. N₂O
Explanation:
1. 1 mol has NA atoms
0.76 mol will have (0.76 . 6.02x10²³) = 4.57x10²³ atoms
2. 6.02x10²³ particles are contained in 1 mol
5.6x10²² particles are contained in (5.6x10²² . 1) / 6.02x10²³ = 0.093 moles
3. 1 mol of water weighs 18 g
0.25 mol . 18g/m = 4.5 grams
4. Molar mass of CaCl₂ = 110.98 g/m
Mol = Mass / molar mass → 48.2 g / 110.98 g/m = 0.434 moles
5. If the volume of Cl₂ at STP (as 1 mol of any gas) is 22.4L, and now we have the half of volume, we will have the half of moles, though. So, we have in the container 0.5 mol of Cl₂
6. 25.9% N and 74.1% O means that in 100 g of compound, we have 25.9 g of N and 74.1 g of O.
25.9 g of N / 14 g/m = 1.85 mol
74.1 g of O / 16 g/m = 4.63 mol
We divide the mol with the lowest value
4.63 mol / 1.85 mol = 2.5
1.85 mol / 1.85 mol = 1
N₂O
 1
1 1. 4.57x10²³ atoms ; 2. 0.093 moles; 3. 4.5 grams ; 4. 0.434 moles , 5. 0.5 mol of Cl₂ ; 6. N₂O
Explanation:
1. 1 mol has NA atoms
0.76 mol will have (0.76 . 6.02x10²³) = 4.57x10²³ atoms
2. 6.02x10²³ particles are contained in 1 mol
5.6x10²² particles are contained in (5.6x10²² . 1) / 6.02x10²³ = 0.093 moles
3. 1 mol of water weighs 18 g
0.25 mol . 18g/m = 4.5 grams
4. Molar mass of CaCl₂ = 110.98 g/m
Mol = Mass / molar mass → 48.2 g / 110.98 g/m = 0.434 moles
5. If the volume of Cl₂ at STP (as 1 mol of any gas) is 22.4L, and now we have the half of volume, we will have the half of moles, though. So, we have in the container 0.5 mol of Cl₂
6. 25.9% N and 74.1% O means that in 100 g of compound, we have 25.9 g of N and 74.1 g of O.
25.9 g of N / 14 g/m = 1.85 mol
74.1 g of O / 16 g/m = 4.63 mol
We divide the mol with the lowest value
4.63 mol / 1.85 mol = 2.5
1.85 mol / 1.85 mol = 1
N₂O
The answer is in the image

Cost of 7 gallons=$24.50
Cost of 1 gallon=24.50/7=3.5
Cost of 15 gallons=15*3.5=52.5
Cost of 15 gallons will be $52.5
The answer is in the image

For every 8 cars there are 7 trucks
Therefore,
Cars:Truck=8:7
Answer is B)8:7
F=ma
where F=force
m=mass
a=acceleration
Here,
F=4300
a=3.3m/s2
m=F/a
=4300/3.3
=1303.03kg
Approximately it is aqual to 1300kg
The answer is in the image

The wood before starting =12 feet
Left wood=6 feet
Wood used till now=12-6=6 feet
Picture frame built till now= 6/(3/4)
=8 pieces
Therefore, till now 8 pieces have been made.

It will provide an instant answer!
