 2
2 B
Step-by-step explanation:
 2
2 Distance = 3.15 miles
Step-by-step explanation:
Given:
Speed = 4.2 mph
Time = 45 mins = 0.75 hr
Required:
Distance = ?
Solution:
Distance = Speed * Time
Distance = 4.2 * 0.75
Distance = 3.15 miles
 2
2 B
Step-by-step explanation:
 2
2 Distance = 3.15 miles
Step-by-step explanation:
Given:
Speed = 4.2 mph
Time = 45 mins = 0.75 hr
Required:
Distance = ?
Solution:
Distance = Speed * Time
Distance = 4.2 * 0.75
Distance = 3.15 miles
 2
2 Explanation:
The equilibrium relevant for this problem is:
H₂PO₄⁻ ↔ HPO₄⁻² + H⁺
The Henderson–Hasselbalch (H-H) equation is needed to solve this problem:
pH= pka + ![log\frac{[A^{-} ]}{[HA]}](/tpl/images/0227/2447/5c113.png)
In this case, [A⁻] = [HPO₄⁻²], [HA] = [H₂PO₄⁻], pH = 7.4; from literature we know that pka=7.21.
We use the H-H equation to describe [HPO₄⁻²] in terms of [H₂PO₄⁻]:
![7.4=7.21+log\frac{[HPO4^{-2} ]}{[H2PO4^{-} ]} \\0.19=log\frac{[HPO4^{-2} ]}{[H2PO4^{-} ]} \\10^{0.19}= \frac{[HPO4^{-2} ]}{[H2PO4^{-} ]} \\1.549*[H2PO4^{-} ]=[HPO4^{-2} ]](/tpl/images/0227/2447/240c2.png)
The problem tells us that the concentration of phosphate is 1 mM, which means:
[HPO₄⁻²] + [H₂PO₄⁻] = 1 mM = 0.001 M
In this equation we can replace [HPO₄⁻²] with the term expressed in the H-H eq:
1.549 * [H₂PO₄⁻] + [H₂PO₄⁻] = 0.001 M
2.549 * [H₂PO₄⁻] = 0.001 M
[H₂PO₄⁻] = 3.923 * 10⁻⁴ M
With the value of [H₂PO₄⁻] we can calculate [HPO₄⁻²]:
[HPO₄⁻²] + 3.923 * 10⁻⁴ M = 0.001 M
[HPO₄⁻²] = 6.077 * 10⁻⁴ M
With the concentrations, the molecular weight, and the volume, we calculate the mass of each reagent:
Mass of NaH₂PO₄ = 3.923 * 10⁻⁴ M * 100 L * 119.98 g/mol = 4.707 gMass of Na₂HPO₄ = 6.077 * 10⁻⁴ M * 100 L * 141.96 g/mol = 8.627 g 1
1 V(2) = 32000*0.8^2 = $20,480
 22
22  7
7 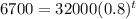
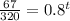
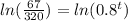
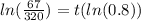
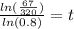
 1
1 7 years
Step-by-step explanation:
Given: The initial price of truck = $32,000
The value of the truck after t years can be represented by the formula ,
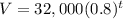
To find the time t in years after which the worth of the truck will be approximately $6700, we need to substitute V=6700 in the equation, we get
Taking log on both sides, we get
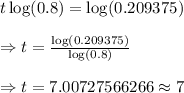
Hence, after 7 years the worth of the truck will be approximately $6700.
 2
2 Explanation:
The equilibrium relevant for this problem is:
H₂PO₄⁻ ↔ HPO₄⁻² + H⁺
The Henderson–Hasselbalch (H-H) equation is needed to solve this problem:
pH= pka + ![log\frac{[A^{-} ]}{[HA]}](/tpl/images/0227/2447/5c113.png)
In this case, [A⁻] = [HPO₄⁻²], [HA] = [H₂PO₄⁻], pH = 7.4; from literature we know that pka=7.21.
We use the H-H equation to describe [HPO₄⁻²] in terms of [H₂PO₄⁻]:
![7.4=7.21+log\frac{[HPO4^{-2} ]}{[H2PO4^{-} ]} \\0.19=log\frac{[HPO4^{-2} ]}{[H2PO4^{-} ]} \\10^{0.19}= \frac{[HPO4^{-2} ]}{[H2PO4^{-} ]} \\1.549*[H2PO4^{-} ]=[HPO4^{-2} ]](/tpl/images/0227/2447/240c2.png)
The problem tells us that the concentration of phosphate is 1 mM, which means:
[HPO₄⁻²] + [H₂PO₄⁻] = 1 mM = 0.001 M
In this equation we can replace [HPO₄⁻²] with the term expressed in the H-H eq:
1.549 * [H₂PO₄⁻] + [H₂PO₄⁻] = 0.001 M
2.549 * [H₂PO₄⁻] = 0.001 M
[H₂PO₄⁻] = 3.923 * 10⁻⁴ M
With the value of [H₂PO₄⁻] we can calculate [HPO₄⁻²]:
[HPO₄⁻²] + 3.923 * 10⁻⁴ M = 0.001 M
[HPO₄⁻²] = 6.077 * 10⁻⁴ M
With the concentrations, the molecular weight, and the volume, we calculate the mass of each reagent:
Mass of NaH₂PO₄ = 3.923 * 10⁻⁴ M * 100 L * 119.98 g/mol = 4.707 gMass of Na₂HPO₄ = 6.077 * 10⁻⁴ M * 100 L * 141.96 g/mol = 8.627 g
It will provide an instant answer!
