The mass of manganese(III) oxide produced is 113.03 g
Explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
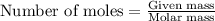 ......(1)
......(1)
Given mass of zinc = 46.8 g
Molar mass of zinc = 65.38 g/mol
Plugging values in equation 1:
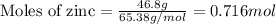
The given chemical equation follows:
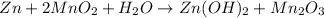
By the stoichiometry of the reaction:
If 1 mole of zinc produces 1 mole of manganese(III) oxide
So, 0.716 moles of zinc will produce = 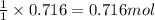 of manganese(III) oxide
of manganese(III) oxide
Molar mass of manganese(III) oxide = 157.87 g/mol
Plugging values in equation 1:
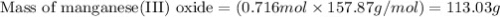
Hence, the mass of manganese(III) oxide produced is 113.03 g
 5
5 Mass = 6.538 g
Explanation:
Given data:
Mass of zinc hydroxide produced = 9.65 g
Mass of zinc required = ?
Solution:
Chemical equation:
Zn + 2MnO₂ + H₂O → Zn(OH)₂ + Mn₂O₃
Number of moles of zinc hydroxide:
Number of moles = mass/molar mass
Number of moles = 9.65 g/ 99.42 g/mol
Number of moles = 0.1 mol
now we will compare the moles of zinc and zinc hydroxide,
Zn(OH)₂ : Zn
1 : 1
0.1 : 0.1
Mass of zinc required:
Mass = number of moles × molar mass
Mass = 0.1 mol × 65.38 g/mol
Mass = 6.538 g
The mass of manganese(III) oxide produced is 113.03 g
Explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
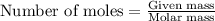 ......(1)
......(1)
Given mass of zinc = 46.8 g
Molar mass of zinc = 65.38 g/mol
Plugging values in equation 1:
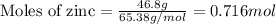
The given chemical equation follows:
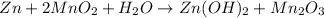
By the stoichiometry of the reaction:
If 1 mole of zinc produces 1 mole of manganese(III) oxide
So, 0.716 moles of zinc will produce = 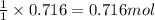 of manganese(III) oxide
of manganese(III) oxide
Molar mass of manganese(III) oxide = 157.87 g/mol
Plugging values in equation 1:
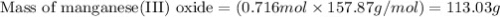
Hence, the mass of manganese(III) oxide produced is 113.03 g
 5
5 Mass = 6.538 g
Explanation:
Given data:
Mass of zinc hydroxide produced = 9.65 g
Mass of zinc required = ?
Solution:
Chemical equation:
Zn + 2MnO₂ + H₂O → Zn(OH)₂ + Mn₂O₃
Number of moles of zinc hydroxide:
Number of moles = mass/molar mass
Number of moles = 9.65 g/ 99.42 g/mol
Number of moles = 0.1 mol
now we will compare the moles of zinc and zinc hydroxide,
Zn(OH)₂ : Zn
1 : 1
0.1 : 0.1
Mass of zinc required:
Mass = number of moles × molar mass
Mass = 0.1 mol × 65.38 g/mol
Mass = 6.538 g
 6
6  6
6  11
11 Following are the solution to the given points:
Explanation:
Oxalic acid volume 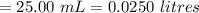
KMnO4 volume 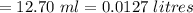
KMnO4 molarity 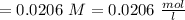
In point a:
Its pink presence after full intake of oxalic acid with attachment to KMnO4 is suggested by the end-point of the process due to the small abundance of KMnO4, As just a self predictor, KMnO4 is used.
In point b:
 molecules mole ratio to
molecules mole ratio to  ions:
ions:
The equilibrium for both the oxalic acid and KMnO4 reaction is suggested:
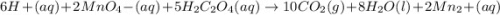
The reaction of 5 mol of oxalic acid is 2 mol 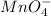 ions
ions
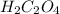 : molecules mole proportion to
: molecules mole proportion to 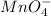 ions:
ions:
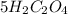 : :
: : 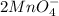
In point c:
The Moles of 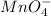 ions reacted with the
ions reacted with the 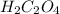 :
:
The molar mass of the solution is the number of solute moles in each volume of water
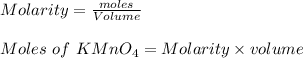
Moles with ions reacted to mol with both the amount of : supplied.
In point d:
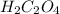 moles in the sample present:
moles in the sample present:
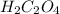 moles = moles
moles = moles 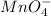 ions
ions  mole ratio
mole ratio
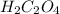 moles in the sample =
moles in the sample = 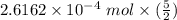
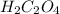 molecules =
molecules = 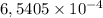 mol are present in the sample
mol are present in the sample
In point e:
Oxalic acid molarity = 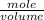
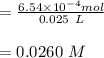
In point f:
Oxalic acid level by mass in the solution:
Oxalic acid mass calculation:
Oxalic acid molar weight = 90.0349 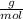 .
.
Oxalic acid mass per liter = oxalic acid moles per liter  molar mass
molar mass
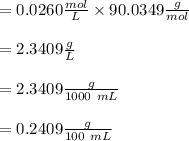
When Oxalic acid solution density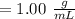
Mass oxalic acid percentage = 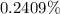
Oxalic acid mass proportion 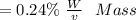
 11
11 Following are the solution to the given points:
Explanation:
Oxalic acid volume 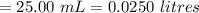
KMnO4 volume 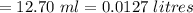
KMnO4 molarity 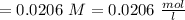
In point a:
Its pink presence after full intake of oxalic acid with attachment to KMnO4 is suggested by the end-point of the process due to the small abundance of KMnO4, As just a self predictor, KMnO4 is used.
In point b:
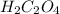 molecules mole ratio to
molecules mole ratio to 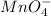 ions:
ions:
The equilibrium for both the oxalic acid and KMnO4 reaction is suggested:
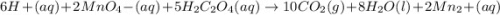
The reaction of 5 mol of oxalic acid is 2 mol 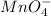 ions
ions
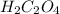 : molecules mole proportion to
: molecules mole proportion to 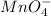 ions:
ions:
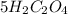 : :
: : 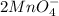
In point c:
The Moles of 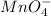 ions reacted with the
ions reacted with the 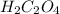 :
:
The molar mass of the solution is the number of solute moles in each volume of water
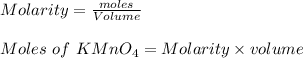
Moles with ions reacted to mol with both the amount of : supplied.
In point d:
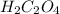 moles in the sample present:
moles in the sample present:
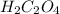 moles = moles
moles = moles 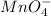 ions
ions  mole ratio
mole ratio
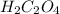 moles in the sample =
moles in the sample = 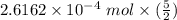
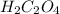 molecules =
molecules = 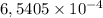 mol are present in the sample
mol are present in the sample
In point e:
Oxalic acid molarity = 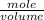
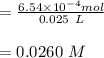
In point f:
Oxalic acid level by mass in the solution:
Oxalic acid mass calculation:
Oxalic acid molar weight = 90.0349 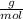 .
.
Oxalic acid mass per liter = oxalic acid moles per liter  molar mass
molar mass
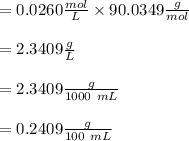
When Oxalic acid solution density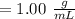
Mass oxalic acid percentage = 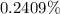
Oxalic acid mass proportion 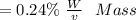
 1
1 In writing a text, these two elements must be present: content and Answer
.Question 2
Refer to the given paragraph below on The Great Wall of China:
What type of description is used in the paragraph?
It is travel writing and it is a location description.
Question text
Which of the following statements is true?
b. You must come up with all the possible ideas from the pre-writing stage.
Question 4
Question text
Refer to the given paragraph below, entitled “Picturing Don Quixote”:
What type of description is used in the paragraph?
Character description and location description
Question 5
Question text
Learning to write is a process.
Question 6
The term that refers to the consequences or events caused by the climax.
The outcome at this point can be predicted.
Question 7
Which of the following questions is not beneficial in exploring your topic?
Select one:
a. Why is it an issue or problem at all?
b. How does the issue relate to other public issues?
c. At what place is the cause or effect of the problem most visible?
d. When is the issue most apparent?
Question 8
Question text
What type of figure of speech is used in the following sentence?
The lady in the water screamed like a banshee.
The correct answer is d. Simile
The writer presented what point of view?
c. First person

It will provide an instant answer!
