Explanation:
Find attach the solution
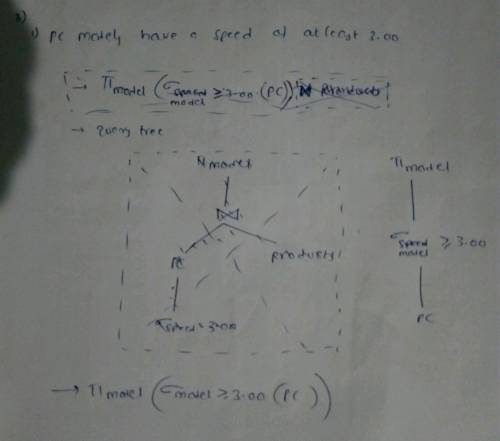
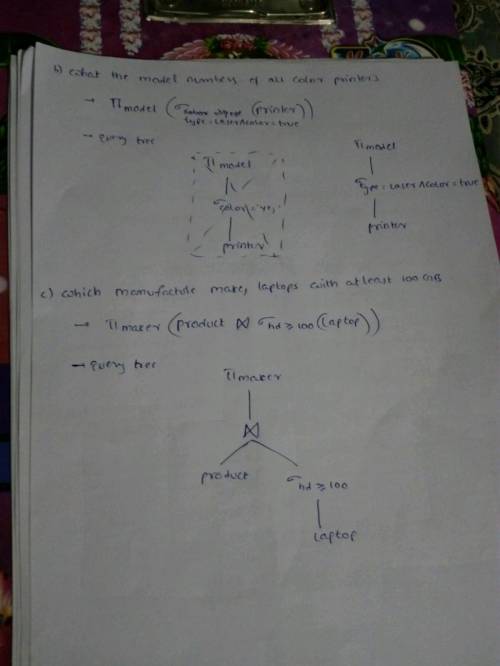
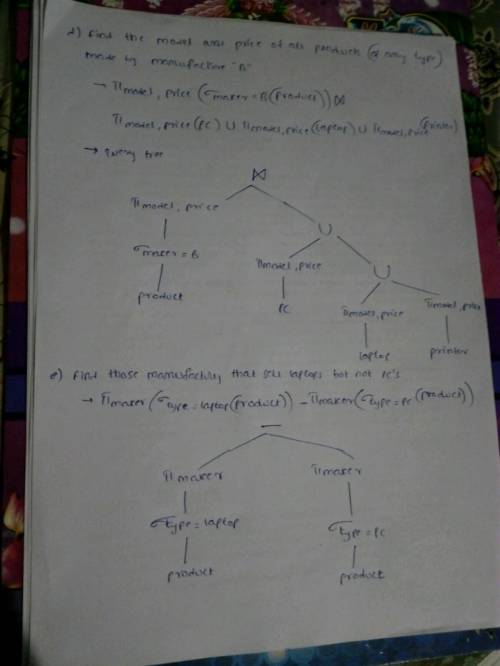
Explanation:
Find attach the solution



 24
24  26
26  24
24  26
26  1
1 d. Both I and II are false
Step-by-step explanation:
When there is a high degree of linear correlation between the predictors the errors are found.
The basic objective of the regression model is to separate the dependent and independent variables. So if the variables have high degree of linear correlation then the multi collinearity causes problems or has errors. It is not necessary that multi collinearity must be present with high degree of linear correlation.
For example we have 3 variable of heat length and time. And all of them have a high degree of correlation. With increase in heat and time the length increases . But for multi collinearity with the increase of time and decrease of heat length does not increase. So this causes errors.
y-hat = 135 + 6x + errors
The linear relationship between height and weight is inexact. The deterministic relation in such cases is then modified to allow the inexact relationship between variables and a non deterministic or probabilistic model is obtained which has error which are unknown random errors.
y- hat= a + bXi + ei (i=1,2,3...)
ei are the unknown random errors.
So both statements are false.
d. Both I and II are false
Step-by-step explanation:
When there is a high degree of linear correlation between the predictors the errors are found.
The basic objective of the regression model is to separate the dependent and independent variables. So if the variables have high degree of linear correlation then the multi collinearity causes problems or has errors. It is not necessary that multi collinearity must be present with high degree of linear correlation.
For example we have 3 variable of heat length and time. And all of them have a high degree of correlation. With increase in heat and time the length increases . But for multi collinearity with the increase of time and decrease of heat length does not increase. So this causes errors.
y-hat = 135 + 6x + errors
The linear relationship between height and weight is inexact. The deterministic relation in such cases is then modified to allow the inexact relationship between variables and a non deterministic or probabilistic model is obtained which has error which are unknown random errors.
y- hat= a + bXi + ei (i=1,2,3...)
ei are the unknown random errors.
So both statements are false.

It will provide an instant answer!
