1. Data:
a) Half-life: 5730 years
b) Final radioactivity: 68%
2. Solution:
a) Determine the number of half-lives undergone
Since, the radioactivity has decreased to 68%, means that the carbon-14 contanined is has been reduced in 32%: 100% - 68% = 32%.32 = 2⁵, meaning that five half-lives have passed since the plant material that formed the parchment fragment died.b) Compute the time of five half-lives:
5 × half-life time = 5 × 5730 years = 28,650 years.c) Round to the nearest hundred:
28,650 years ≈ 28,700 yearsAnd that is the age of the parchment.
1. Data:
a) Half-life: 5730 years
b) Final radioactivity: 68%
2. Solution:
a) Determine the number of half-lives undergone
Since, the radioactivity has decreased to 68%, means that the carbon-14 contanined is has been reduced in 32%: 100% - 68% = 32%.32 = 2⁵, meaning that five half-lives have passed since the plant material that formed the parchment fragment died.b) Compute the time of five half-lives:
5 × half-life time = 5 × 5730 years = 28,650 years.c) Round to the nearest hundred:
28,650 years ≈ 28,700 yearsAnd that is the age of the parchment.
2500 years
Step-by-step explanation:
I'm not quite sure if I'm doing this right myself but I'll give it a shot.
We use this formula to find half-life but we can just plug in the numbers we know to find t.
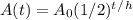
We know half-life is 5730 years and that the parchment has retained 74% of its Carbon-14. For 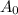 let's just assume that there are 100 original atoms of Carbon-14 and for A(t) let's assume there are 74 Carbon-14 atoms AFTER the amount of time has passed. That way, 74% of the C-14 still remains as 74/100 is 74%. Not quite sure how to explain it but I hope you get it. h is the last variable we need to know and it's just the half-life, which has been given to us already, 5730 years, so now we have this.
let's just assume that there are 100 original atoms of Carbon-14 and for A(t) let's assume there are 74 Carbon-14 atoms AFTER the amount of time has passed. That way, 74% of the C-14 still remains as 74/100 is 74%. Not quite sure how to explain it but I hope you get it. h is the last variable we need to know and it's just the half-life, which has been given to us already, 5730 years, so now we have this.
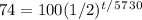
Now, solve. First, divide by 100.
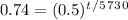
Take the log of everything
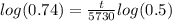
Divide the entire equation by log (0.5) and multiply the entire equation by 5730 to isolate the t and get
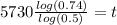
Use your calculator to solve that giant mess for t and you'll get that t is roughly 2489.128182 years. Round that to the nearest hundred years, and you'll find the hopefully correct answer is 2500 years.
Really hope that all the equations that I wrote came out good and that that's right, this is definitely the longest answer I've ever written.
Explanation:
These steps explain how you estimate the age of the parchment:
1) Carbon - 14 half-life: τ = 5730 years
2) Number of half-lives elapsed: n
3) Age of the parchment = τ×n = 5730×n years = 5730n
4) Exponential decay:
The ratio of the final amount of the radioactive isotope C-14 to the initial amount of the same is one half (1/2) raised to the number of half-lives elapsed (n):
A / Ao = (1/2)ⁿ5) The parchment fragment had about 74% as much C-14 radioactivity as does plant material on Earth today:
⇒ A / Ao = 74% = 0.74⇒ A / Ao = 0.74 = (1/2)ⁿ ⇒ ln (0.74) = n ln (1/2) [apply natural logarithm to both sides]⇒ n = ln (1/2) / ln (0.74)⇒ n ≈ - 0.693 / ( - 0.301) = 2.30Hence, 2.30 half-lives have elapsed and the age of the parchment is:
τ×n = 5730n = 5730 (2.30) = 13,179 years Round to the nearest hundred years: 13,200 years 1
1  years
years
Explanation:
Given-
Half life of 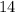 C
C 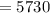 years
years
As we know -
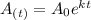
Where
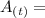 Mass of radioactive carbon after a time period "t"
Mass of radioactive carbon after a time period "t"
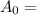 initial mass of radioactive carbon
initial mass of radioactive carbon
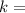 radioactive decay constant
radioactive decay constant
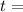 time
time
First we will find the value of "k"
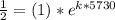
On solving, we get -
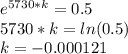
Now, when mass of 14C becomes 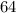 % of the plant material on earth today, then its age would be
% of the plant material on earth today, then its age would be
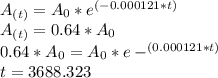 years
years
B
Explanation:
A detailed solution to the problem is shown in the image attached. You could study it. The half life, and percentage of C-14 compared to present living sample was given as 13% of that of a living sample.

The answer is in the image

The answer is in the image

The answer is in the image

The solution is given in the image below


It will provide an instant answer!
