 3
3 9514 1404 393
EF = 1.98AB = 16.25HI = 12.22Step-by-step explanation:
The mnemonic SOH CAH TOA is useful for these. It reminds you of the relations between sides and trig functions in a right triangle.
__
1. The side of interest is opposite the given angle. The given side is the hypotenuse.
Sin = Opposite/Hypotenuse
sin(26°) = EF/DF = EF/4.5
EF = 4.5·sin(26°) = 4.5·0.44 = 1.98 . . . units
__
2. The given side is adjacent to the given angles. The desired length is that of the hypotenuse.
Cos = Adjacent/Hypotenuse
cos(52°) = AC/AB = 10.01/c
c = 10.01/cos(52°) = 10.01/0.616 ≈ 16.25 . . . units
__
3. The given side is opposite the given angle, and the desired side length is adjacent to the given angle.
Tan = Opposite/Adjacent
tan(42°) = 11/HI
HI = 11/tan(42°) = 11/0.90 = 12.22 . . . units
 3
3 9514 1404 393
EF = 1.98AB = 16.25HI = 12.22Step-by-step explanation:
The mnemonic SOH CAH TOA is useful for these. It reminds you of the relations between sides and trig functions in a right triangle.
__
1. The side of interest is opposite the given angle. The given side is the hypotenuse.
Sin = Opposite/Hypotenuse
sin(26°) = EF/DF = EF/4.5
EF = 4.5·sin(26°) = 4.5·0.44 = 1.98 . . . units
__
2. The given side is adjacent to the given angles. The desired length is that of the hypotenuse.
Cos = Adjacent/Hypotenuse
cos(52°) = AC/AB = 10.01/c
c = 10.01/cos(52°) = 10.01/0.616 ≈ 16.25 . . . units
__
3. The given side is opposite the given angle, and the desired side length is adjacent to the given angle.
Tan = Opposite/Adjacent
tan(42°) = 11/HI
HI = 11/tan(42°) = 11/0.90 = 12.22 . . . units
9514 1404 393
AB = 16.25HI = 12.22Step-by-step explanation:
The mnemonic SOH CAH TOA reminds you of the relevant relationships.
__
1. Cos = Adjacent/Hypotenuse
cos(52°) = AC/AB
AB = AC/cos(52°) = 10.01/0.616
AB = 16.25
__
2. Tan = Opposite/Adjacent
tan(42°) = GH/HI = 11/HI
HI = 11/tan(42°) = 11/0.90
HI ≈ 12.22
9514 1404 393
AB = 16.25HI = 12.22Step-by-step explanation:
The mnemonic SOH CAH TOA reminds you of the relevant relationships.
__
1. Cos = Adjacent/Hypotenuse
cos(52°) = AC/AB
AB = AC/cos(52°) = 10.01/0.616
AB = 16.25
__
2. Tan = Opposite/Adjacent
tan(42°) = GH/HI = 11/HI
HI = 11/tan(42°) = 11/0.90
HI ≈ 12.22
1- c. Two-way ANOVA
2- a. 1
3- c. 78.1%
4- 48.9
Step-by-step explanation:
ANOVA is a statistical technique designed to test mean of one or more quantitative populations. In two-way ANOVA it equals the block mean. Column block means square is three-way ANOVA.
Oxygen intake rate varies for different people. It also depends on the age factor. Oxygen intake rate is indicator of normal function of cells. A 48 year old man breath slower than a younger man. The babies breathe faster than adults.
1- c. Two-way ANOVA
2- a. 1
3- c. 78.1%
4- 48.9
Step-by-step explanation:
ANOVA is a statistical technique designed to test mean of one or more quantitative populations. In two-way ANOVA it equals the block mean. Column block means square is three-way ANOVA.
Oxygen intake rate varies for different people. It also depends on the age factor. Oxygen intake rate is indicator of normal function of cells. A 48 year old man breath slower than a younger man. The babies breathe faster than adults.
 4
4 The ppm carbon in the seawater is 104.01.
Explanation:
Mass of seawater = 6.234 g
Mass of carbon dioxide gas produced = 2.378 mg = 0.002378 g
1 mg = 0.001 g
Moles of carbon dioxide gas = 
1 mol of carbon dioxde gas has 1 mole pf carbon atoms,then  will have ;
will have ;
 of carbon
of carbon
Mass of  of carbon ;
of carbon ;

To calculate the ppm of oxygen in sea water, we use the equation:
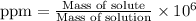
(Both the masses are in grams)

The ppm carbon in the seawater is 104.01.
 4
4 The ppm carbon in the seawater is 104.01.
Explanation:
Mass of seawater = 6.234 g
Mass of carbon dioxide gas produced = 2.378 mg = 0.002378 g
1 mg = 0.001 g
Moles of carbon dioxide gas = 
1 mol of carbon dioxde gas has 1 mole pf carbon atoms,then  will have ;
will have ;
 of carbon
of carbon
Mass of  of carbon ;
of carbon ;

To calculate the ppm of oxygen in sea water, we use the equation:
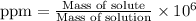
(Both the masses are in grams)

The ppm carbon in the seawater is 104.01.
For 1 flavor there are 9 topping
Therefore, for 5 different flavors there will be 5*9 choices
No of choices= 5*9
=45
The answer is in the image
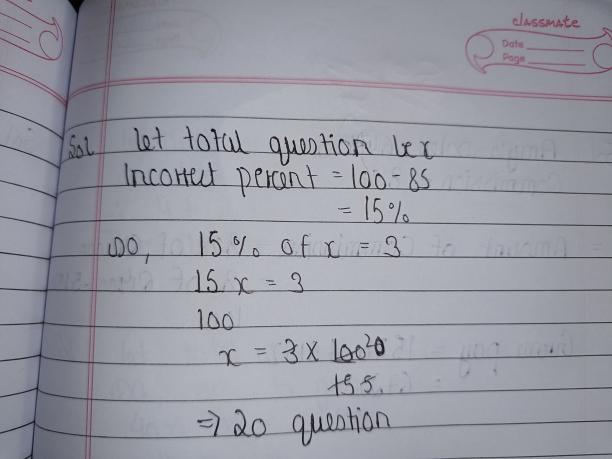

It will provide an instant answer!
