 12
12  26
26  12
12 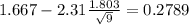
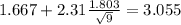
So on this case the 95% confidence interval would be given by (0.2789;3.055)
The 95% confidence interval to judge whether the two indeners result in different measurements is?
Yes the confidence interval not contains the value 0 so we can conclude that the values for Diamond are significantly higher than the values for Steel Ball at 5% of significance.
Step-by-step explanation:
We have the following dataset:
specimen 1 2 3 4 5 6 7 8 9
Steel Ball 51 57 61 70 68 54 65 51 53
Diamond 53 55 63 74 69 56 68 51 56
If we calculate the differences diamond-steel ball we have this datase:
d: 2, -2, 2, 4, 1, 2, 3, 0, 3
The second step is calculate the mean difference
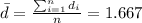
The third step would be calculate the standard deviation for the differences, and we got:
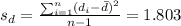
A confidence interval is "a range of values that’s likely to include a population value with a certain degree of confidence. It is often expressed a % whereby a population means lies between an upper and lower interval".
The margin of error is the range of values below and above the sample statistic in a confidence interval.
Normal distribution, is a "probability distribution that is symmetric about the mean, showing that data near the mean are more frequent in occurrence than data far from the mean".
The confidence interval for the mean is given by the following formula:
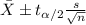 (1)
(1)
In order to calculate the critical value 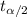 we need to find first the degrees of freedom, given by:
we need to find first the degrees of freedom, given by:
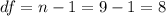
Since the confidence is 0.95 or 95%, the value of 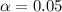 and
and 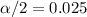 , and we can use excel, a calculator or a table to find the critical value. The excel command would be: "=-T.INV(0.025,9)".And we see that
, and we can use excel, a calculator or a table to find the critical value. The excel command would be: "=-T.INV(0.025,9)".And we see that 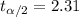 .
.
Now we have everything in order to replace into formula (1):
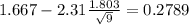
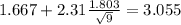
So on this case the 95% confidence interval would be given by (0.2789;3.055)
The 95% confidence interval to judge whether the two indeners result in different measurements is?
Yes the confidence interval not contains the value 0 so we can conclude that the values for Diamond are significantly higher than the values for Steel Ball at 5% of significance.
Check the explanation
Step-by-step explanation:
Let X denotes steel ball and Y denotes diamond
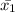 = 1/9( 50+57+......+51+53)
= 1/9( 50+57+......+51+53)
=530/9
=58.89
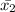 = 1/9( 52+ 56+....+ 51+ 56)
= 1/9( 52+ 56+....+ 51+ 56)
=543/9
=60.33
difference = d =(60.33- 58.89)
=1.44
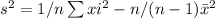
s12 = 1/9( 502+572+......+512+532) -9/8 (58.89)2
=31686/8 - 9/8( 3468.03)
=3960.75 - 3901.53
=59.22
s1 = 7.69
s22 = 1/9( 522+ 562+....+ 512+ 562) -9/8 (60.33)2
=33295/8 - 9/8 (3640.11)
=4161.875 - 4095.12
=66.75
s2 =8.17
sample standard deviation for difference is
s=![\sqrt{[(n1-1)s_1^2+ (n2-1)s_2^2]/(n1+n2-2)}](/tpl/images/0658/6333/27051.png)
= ![\sqrt{[(9-1)*59.22+ (9-1)*66.75]/(9+9-2)}](/tpl/images/0658/6333/52363.png)
= 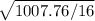
=7.93
sd = 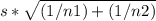
=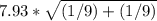
=7.93* 0.47
=3.74
For 95% confidence level 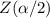 =1.96
=1.96
confidence interval is
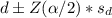
=(1.44 - 1.96* 3.75 , 1.44+1.96* 3.75)
=(1.44 - 7.35 , 1.44 + 7.35)
=(-2.31, 8.79)
There is sufficient evidence to conclude that the two indenters produce different hardness readings.
pH = 12.7
Explanation:
First, we have to calculate the [Ca²⁺] in a solution of about 250 ppm CaCO₃.
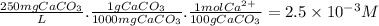
Now, let's consider the dissolution of Ca(OH)₂ in water.
Ca(OH)₂(s) ⇄ Ca²⁺(aq) + 2 OH⁻(aq)
The solubility product Ksp is:
Ksp = [Ca²⁺] × [OH⁻]²
[OH⁻] = √(Ksp/[Ca²⁺]) = √(6.5 × 10⁻⁶/2.5 × 10⁻³) = 5.1 × 10⁻² M
Finally, we can calculate pOH and pH.
pOH = -log [OH⁻] = -log (5.1 × 10⁻²) = 1.3
pH + pOH = 14 ⇒ pH = 14 - pOH = 14 - 1.3 = 12.7
 13
13 i would say (c)
Explanation:
 4
4 Im pretty sure its B
Explanation:
hopet this helped! :D
 4
4 Im pretty sure its B
Explanation:
hopet this helped! :D
 6
6 Die stone i think im no sure

It will provide an instant answer!
