1.133 kPa is the average pressure exerted by the molecules on the walls of the container.
Explanation:
Side of the cubic box = s = 20.0 cm
Volume of the box ,V= 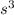
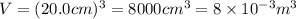
Root mean square speed of the of helium molecule : 200m/s
The formula used for root mean square speed is:
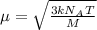
where,
= root mean square speed
k = Boltzmann’s constant = 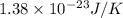
T = temperature = 370 K
M = mass helium = 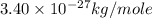
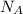 = Avogadro’s number =
= Avogadro’s number = 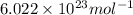
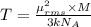
Moles of helium gas = n
Number of helium molecules = N =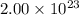
N = 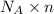
Ideal gas equation:
PV = nRT
Substitution of values of T and n from above :
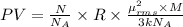
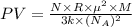
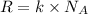
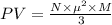
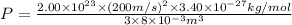
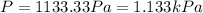
(1 Pa = 0.001 kPa)
1.133 kPa is the average pressure exerted by the molecules on the walls of the container.
1.133 kPa is the average pressure exerted by the molecules on the walls of the container.
Explanation:
Side of the cubic box = s = 20.0 cm
Volume of the box ,V= 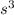
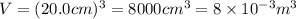
Root mean square speed of the of helium molecule : 200m/s
The formula used for root mean square speed is:
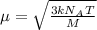
where,
= root mean square speed
k = Boltzmann’s constant = 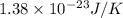
T = temperature = 370 K
M = mass helium = 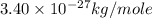
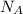 = Avogadro’s number =
= Avogadro’s number = 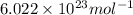
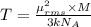
Moles of helium gas = n
Number of helium molecules = N =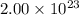
N = 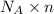
Ideal gas equation:
PV = nRT
Substitution of values of T and n from above :
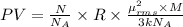
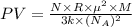
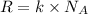
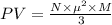
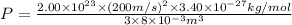
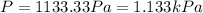
(1 Pa = 0.001 kPa)
1.133 kPa is the average pressure exerted by the molecules on the walls of the container.
4. The combined volume of the Ar atoms is too large to be negligible compared with the total volume of the container.
Explanation:
Deviations from ideality are due to intermolecular forces and to the nonzero volume of the molecules themselves. At infinite volume, the volume of the molecules themselves is negligible compared with the infinite volume the gas occupies.
However, the volume occupied by the gas molecules must be taken into account. Each molecule does occupy a finite, although small, intrinsic volume.
The non-zero volume of the molecules implies that instead of moving in a given volume V they are limited to doing so in a smaller volume. Thus, the molecules will be closer to each other and repulsive forces will dominate, resulting in greater pressure than the one calculated with the ideal gas law, that means, without considering the volume occupied by the molecules.
4. The combined volume of the Ar atoms is too large to be negligible compared with the total volume of the container.
Explanation:
Deviations from ideality are due to intermolecular forces and to the nonzero volume of the molecules themselves. At infinite volume, the volume of the molecules themselves is negligible compared with the infinite volume the gas occupies.
However, the volume occupied by the gas molecules must be taken into account. Each molecule does occupy a finite, although small, intrinsic volume.
The non-zero volume of the molecules implies that instead of moving in a given volume V they are limited to doing so in a smaller volume. Thus, the molecules will be closer to each other and repulsive forces will dominate, resulting in greater pressure than the one calculated with the ideal gas law, that means, without considering the volume occupied by the molecules.
8563732.58906 Pa
3992793.23326 Pa
5708.00923 J
Explanation:
V = Volume
N = Number of molecules = 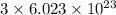
T = Temperature = 300 K
b = 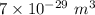
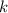 = Boltzmann constant =
= Boltzmann constant = 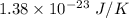
P = Pressure
We have the equation
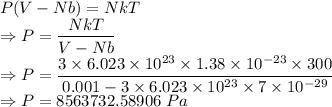
The pressure is 8563732.58906 Pa
For isothermal expansion
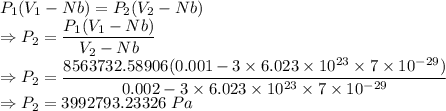
The pressure is 3992793.23326 Pa
Work done is given by
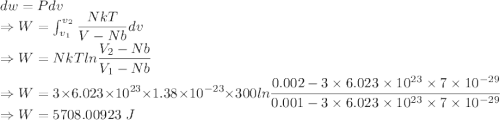
The work done is 5708.00923 J
8563732.58906 Pa
3992793.23326 Pa
5708.00923 J
Explanation:
V = Volume
N = Number of molecules = 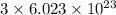
T = Temperature = 300 K
b = 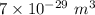
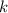 = Boltzmann constant =
= Boltzmann constant = 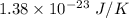
P = Pressure
We have the equation
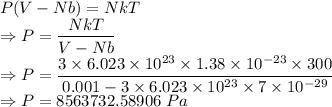
The pressure is 8563732.58906 Pa
For isothermal expansion
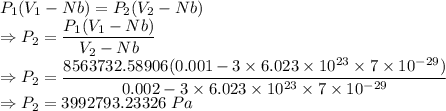
The pressure is 3992793.23326 Pa
Work done is given by
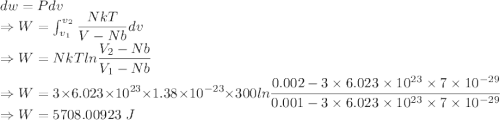
The work done is 5708.00923 J
 6
6 These are four questions, each with its complete answer.
Question 1. If the temperature of a gas remains constant, then the pressure of the gas will increase if the a. mass of the gas molecules decreases. b. diffusion of the gas molecules increases. c. size of the container is decreased. d. number of gas molecules in the container is decreased.
Option c. the size of the container is decreased.Explanation:
At constant temperature, the pressure of a gas has the following relations with other variables:
Amount of gas: direct proportion, the greater the amount of particles of the gas, molecules or atoms, the greater the pressure of the gas.Then, as long as the number of molecules in the gas does not change, a decrease in the mass of the gas molecules (option a) does not modify the pressure, which makes that the option a. is not valid.
Option b, diffusion of the gas molecules increases, means that some molecules will abandon the container. So, following the direct proportion of the pressure with the number of molecules, this option means a decrease of the pressure, and you discard it.
Following the same reasoning, the option d, number of gas molceules in the container is decreased, also means a decrease of the pressure, and this option is discarded.
Volume: as per Boyle's law, the volume and the pressure of a gas are in inversed relation. Then, the option c., size of the container is decreased, indeed means the increase of the pressure, and this is the correct option.Question 2. When Gay-Lussac’s law of combining volumes holds, which of the following can be expressed in ratios of small whole numbers? a. pressures before and after reaction b. volumes of gaseous reactants and products c. kelvin temperatures d. molar masses of products and molar masses of reactants
option b. volumes of gaseous reactants and productsExplanation:
Gay-Lussac’s law of combning volumes states that, at constant temperature and pressure, when gases combine the volumes are in the ratio of simple whole numbers.
Hence, it is not pressures before and after reaction (option a), kelvin temperatures (option c), or molar masses of products and molar masses of reactants (option d) what holds.
It is volumes of gaseous reactants and products (option b) what holds.
Question 3. Equal volumes of ideal gases at the same temperature and pressure contain equal numbers of a. protons. b. ions. c. particles. d. electrons.
option c. particles.Explanation:
This is a direct deduction from Avogadro's principle: no matter the size of the particles, either single atoms, or small or large molecules, at the same temperature and pressure, equal volume of gases contain the same number of particles (atoms or molecules).
That is why it is stated that at 1 atm and 0°C, the volume of 1 mole of any gas is approximately 22.4 liter.
Question 4. At constant temperature and pressure, the volume of a gas is directly proportional to its a. molar mass. b. number of moles. c. density at STP. d. rate of diffusion.
option b. number of moles.Explanation:
As explained on the answer to the question 4, Avogadro's law states that at constant temperature and pressure, the volume of a gas is directly proportional to its number of particles.
Moles is a unit of amount of particles. One mole is equal to 6.022 × 10²³ particles (atoms or moles, in the case of gases).
You can also reason from the ideal gas equation:
pV = nRT ⇒ n = (pV) / (RT) = V (p / RT)Then, since (p / RT) is constant, p is directly proportional to V. 6
6 These are four questions, each with its complete answer.
Question 1. If the temperature of a gas remains constant, then the pressure of the gas will increase if the a. mass of the gas molecules decreases. b. diffusion of the gas molecules increases. c. size of the container is decreased. d. number of gas molecules in the container is decreased.
Option c. the size of the container is decreased.Explanation:
At constant temperature, the pressure of a gas has the following relations with other variables:
Amount of gas: direct proportion, the greater the amount of particles of the gas, molecules or atoms, the greater the pressure of the gas.Then, as long as the number of molecules in the gas does not change, a decrease in the mass of the gas molecules (option a) does not modify the pressure, which makes that the option a. is not valid.
Option b, diffusion of the gas molecules increases, means that some molecules will abandon the container. So, following the direct proportion of the pressure with the number of molecules, this option means a decrease of the pressure, and you discard it.
Following the same reasoning, the option d, number of gas molceules in the container is decreased, also means a decrease of the pressure, and this option is discarded.
Volume: as per Boyle's law, the volume and the pressure of a gas are in inversed relation. Then, the option c., size of the container is decreased, indeed means the increase of the pressure, and this is the correct option.Question 2. When Gay-Lussac’s law of combining volumes holds, which of the following can be expressed in ratios of small whole numbers? a. pressures before and after reaction b. volumes of gaseous reactants and products c. kelvin temperatures d. molar masses of products and molar masses of reactants
option b. volumes of gaseous reactants and productsExplanation:
Gay-Lussac’s law of combning volumes states that, at constant temperature and pressure, when gases combine the volumes are in the ratio of simple whole numbers.
Hence, it is not pressures before and after reaction (option a), kelvin temperatures (option c), or molar masses of products and molar masses of reactants (option d) what holds.
It is volumes of gaseous reactants and products (option b) what holds.
Question 3. Equal volumes of ideal gases at the same temperature and pressure contain equal numbers of a. protons. b. ions. c. particles. d. electrons.
option c. particles.Explanation:
This is a direct deduction from Avogadro's principle: no matter the size of the particles, either single atoms, or small or large molecules, at the same temperature and pressure, equal volume of gases contain the same number of particles (atoms or molecules).
That is why it is stated that at 1 atm and 0°C, the volume of 1 mole of any gas is approximately 22.4 liter.
Question 4. At constant temperature and pressure, the volume of a gas is directly proportional to its a. molar mass. b. number of moles. c. density at STP. d. rate of diffusion.
option b. number of moles.Explanation:
As explained on the answer to the question 4, Avogadro's law states that at constant temperature and pressure, the volume of a gas is directly proportional to its number of particles.
Moles is a unit of amount of particles. One mole is equal to 6.022 × 10²³ particles (atoms or moles, in the case of gases).
You can also reason from the ideal gas equation:
pV = nRT ⇒ n = (pV) / (RT) = V (p / RT)Then, since (p / RT) is constant, p is directly proportional to V. 1
1 A. Molecules have finite volume.
Explanation:
Gases deviate from the ideal gas law at high pressures because its molecules have a finite volume.
Real gases have a finite volume which enables more interaction between the molecules while ideal gases are assumed not to have a finite volume or occupy space which is why it lacks any form of interaction between its molecules.
This difference is the deviation between the real and ideal gases.
 1
1 A. Molecules have finite volume.
Explanation:
Gases deviate from the ideal gas law at high pressures because its molecules have a finite volume.
Real gases have a finite volume which enables more interaction between the molecules while ideal gases are assumed not to have a finite volume or occupy space which is why it lacks any form of interaction between its molecules.
This difference is the deviation between the real and ideal gases.

It will provide an instant answer!
