1. Empirical formula is MgO
2. 2Mg + O₂ --> 2MgO
3. 3Mg + N₂ ---> Mg₃N₂
4. Mg₃N₂ + 6H₂O > 3Mg(OH)₂ + 2NH₃
5. Mg(OH)₂ ---> MgO + H₂O
Explanation:
1. Empirical formula:
mass of magnesium oxide = 0.3327 g; mass of magnesium = 0.2000 g
mass of oxygen = 0.3317 g - 0.2000 g = 0.1317 g
molar mass of magnesium = 24, molar mass of oxygen = 16
molar ratio of magnesium to oxygen is then calculated;
magnesium = 0.2000/24 = 0.0083 : oxygen = 0.1317/16 = 0.0082
magnesium = 0.0083/0.0082 = 1 : oxygen = 0.0082/0.0082 = 1
molar ratio of magnesium to oxygen = 1 : 1
Therefore, empirical formula is MgO
2. The reaction of magnesium with molecular oxygen produces magnesium oxide as shown by the equation below:
2Mg + O₂ --> 2MgO
3. The reaction of magnesium with molecular nitrogen produces magnesium nitride as shown by the equation below:
3Mg + N₂ ---> Mg₃N₂
4. The reaction of magnesium nitride with water produces magnesium hydroxide and ammonia gas as shown by the equation below:
Mg₃N₂ + 6H₂O > 3Mg(OH)₂ + 2NH₃
5. The products of the reaction of heating magnesium hydroxide are magnesium oxide and water as shown by the equation below:
Mg(OH)₂ ---> MgO + H₂O
1. Empirical formula is MgO
2. 2Mg + O₂ --> 2MgO
3. 3Mg + N₂ ---> Mg₃N₂
4. Mg₃N₂ + 6H₂O > 3Mg(OH)₂ + 2NH₃
5. Mg(OH)₂ ---> MgO + H₂O
Explanation:
1. Empirical formula:
mass of magnesium oxide = 0.3327 g; mass of magnesium = 0.2000 g
mass of oxygen = 0.3317 g - 0.2000 g = 0.1317 g
molar mass of magnesium = 24, molar mass of oxygen = 16
molar ratio of magnesium to oxygen is then calculated;
magnesium = 0.2000/24 = 0.0083 : oxygen = 0.1317/16 = 0.0082
magnesium = 0.0083/0.0082 = 1 : oxygen = 0.0082/0.0082 = 1
molar ratio of magnesium to oxygen = 1 : 1
Therefore, empirical formula is MgO
2. The reaction of magnesium with molecular oxygen produces magnesium oxide as shown by the equation below:
2Mg + O₂ --> 2MgO
3. The reaction of magnesium with molecular nitrogen produces magnesium nitride as shown by the equation below:
3Mg + N₂ ---> Mg₃N₂
4. The reaction of magnesium nitride with water produces magnesium hydroxide and ammonia gas as shown by the equation below:
Mg₃N₂ + 6H₂O > 3Mg(OH)₂ + 2NH₃
5. The products of the reaction of heating magnesium hydroxide are magnesium oxide and water as shown by the equation below:
Mg(OH)₂ ---> MgO + H₂O
 9
9 Explanation:
Charges on both magnesium and oxygen is 2. Though opposite in sign, they have equal charges so, both of them will be cancelled by each other.
As a result, formula of magnesium oxide is MgO and not 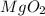 .
.
The student write the equation as 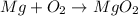 , it is not correct.
, it is not correct.
Therefore, given equation will be balanced as follows.
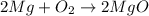
Since, number of atoms on both reactant and product side are equal. Hence, this equation is completely balanced.
 9
9 Explanation:
Charges on both magnesium and oxygen is 2. Though opposite in sign, they have equal charges so, both of them will be cancelled by each other.
As a result, formula of magnesium oxide is MgO and not 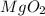 .
.
The student write the equation as 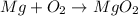 , it is not correct.
, it is not correct.
Therefore, given equation will be balanced as follows.
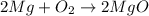
Since, number of atoms on both reactant and product side are equal. Hence, this equation is completely balanced.
 4
4 Thus the correct coefficient for sodium chloride is 2.
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The balanced chemical reaction is:
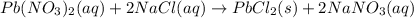
Thus the correct coefficient for sodium chloride is 2.
 4
4 Thus the correct coefficient for sodium chloride is 2.
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The balanced chemical reaction is:
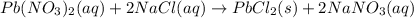
Thus the correct coefficient for sodium chloride is 2.
 3
3 The correct option is: C. 2Ca + O₂ = 2CaO
Explanation:
Calcium is a s-block element, belonging to the group 2 and period 4 of the periodic table.
It is an alkaline earth metal having 2 valence electrons in its outermost s-orbital. So, calcium generally exists in +2 oxidation state in its compounds.
When calcium (Ca) reacts with oxygen (O₂), calcium oxide or quicklime (CaO) is formed.
The balanced chemical equation is: 2Ca + O₂ → 2CaO
 3
3 The correct option is: C. 2Ca + O₂ = 2CaO
Explanation:
Calcium is a s-block element, belonging to the group 2 and period 4 of the periodic table.
It is an alkaline earth metal having 2 valence electrons in its outermost s-orbital. So, calcium generally exists in +2 oxidation state in its compounds.
When calcium (Ca) reacts with oxygen (O₂), calcium oxide or quicklime (CaO) is formed.
The balanced chemical equation is: 2Ca + O₂ → 2CaO
 2
2 See below
Explanation:
Coefficient on Fe₃(PO₄)₂ is incorrect => such means sum of mass reactants does not equal sum mass of products. Violates Law of Conservation of Matter in chemical reactions.
Correct balance is ...
6LiBr + Fe₃(PO₄)₂ => 2Li₃PO₄ + 3FeBr₂
 2
2 See below
Explanation:
Coefficient on Fe₃(PO₄)₂ is incorrect => such means sum of mass reactants does not equal sum mass of products. Violates Law of Conservation of Matter in chemical reactions.
Correct balance is ...
6LiBr + Fe₃(PO₄)₂ => 2Li₃PO₄ + 3FeBr₂

It will provide an instant answer!
