The answer to your question is He needs 50 ml of 2.0 M HCl.
Explanation:
Data
Volume 2 = 400 ml
[HCl] 2 = 0.25 M
[HCl] 1 = 2.0 M
Volume 1 = ?
To solve this problem use the dilution formula
Formula
V₁C₁ = V₂C₂
Solve for V₁
V₁ = V₂C₂ / C₁
Substitution
V₁ = (400)(0.25) / 2
Simplification
V₁ = 100 / 2
Result
V₁ = 50 ml
The answer to your question is He needs 50 ml of 2.0 M HCl.
Explanation:
Data
Volume 2 = 400 ml
[HCl] 2 = 0.25 M
[HCl] 1 = 2.0 M
Volume 1 = ?
To solve this problem use the dilution formula
Formula
V₁C₁ = V₂C₂
Solve for V₁
V₁ = V₂C₂ / C₁
Substitution
V₁ = (400)(0.25) / 2
Simplification
V₁ = 100 / 2
Result
V₁ = 50 ml
 9
9 418.6 mL of stock solution of HCl will be needed to prepare the required amount of solution.
Explanation:
To calculate the volume of stock solution required to prepare the HCl solution, we use the following equation:
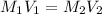
where,
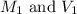 are the molarity and volume of Stock HCl solution
are the molarity and volume of Stock HCl solution
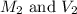 are the molarity and volume of required HCl solution.
are the molarity and volume of required HCl solution.
We are given:
Conversion factor: 1L = 1000mL
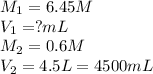
Putting values in above equation, we get:
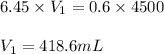
Hence, 418.6 mL of stock solution of HCl will be needed to prepare the required amount of solution.
418.6 mL of stock solution of HCl will be needed to prepare the required amount of solution.
Explanation:
To calculate the volume of stock solution required to prepare the HCl solution, we use the following equation:
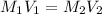
where,
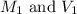 are the molarity and volume of Stock HCl solution
are the molarity and volume of Stock HCl solution
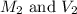 are the molarity and volume of required HCl solution.
are the molarity and volume of required HCl solution.
We are given:
Conversion factor: 1L = 1000mL
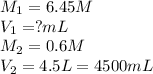
Putting values in above equation, we get:
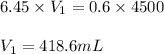
Hence, 418.6 mL of stock solution of HCl will be needed to prepare the required amount of solution.
Answer : The pH of the solution is, 1.88
Explanation : Given,
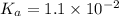
Concentration of 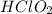 = 0.35 M
= 0.35 M
Concentration of 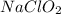 = 0.29 M
= 0.29 M
First we have to calculate the value of 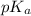 .
.
The expression used for the calculation of 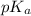 is,
is,
![pK_a=-\log [K_a]](/tpl/images/0067/9400/2d95a.png)
Now put the value of 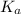 in this expression, we get:
in this expression, we get:
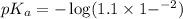
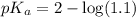
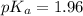
Now we have to calculate the pH of the solution.
Using Henderson Hesselbach equation :
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0067/9400/e961a.png)
![pH=pK_a+\log \frac{[NaClO_2]}{[HClO_2]}](/tpl/images/0067/9400/a8df0.png)
Now put all the given values in this expression, we get:
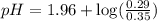
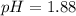
Therefore, the pH of the solution is, 1.88
Answer : The pH of the solution is, 1.88
Explanation : Given,
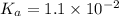
Concentration of 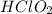 = 0.35 M
= 0.35 M
Concentration of 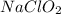 = 0.29 M
= 0.29 M
First we have to calculate the value of 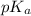 .
.
The expression used for the calculation of 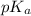 is,
is,
![pK_a=-\log [K_a]](/tpl/images/0067/9400/2d95a.png)
Now put the value of 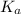 in this expression, we get:
in this expression, we get:
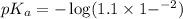
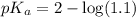
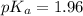
Now we have to calculate the pH of the solution.
Using Henderson Hesselbach equation :
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0067/9400/e961a.png)
![pH=pK_a+\log \frac{[NaClO_2]}{[HClO_2]}](/tpl/images/0067/9400/a8df0.png)
Now put all the given values in this expression, we get:
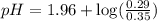
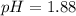
Therefore, the pH of the solution is, 1.88
V = 0.5 L
Explanation:
Given that,
The volume of HCl, V = 2 M
We need to find the volume of 2.0M HCl is needed to prepare 0.50L of a 0.75M solution.
Let n be the number of moles of HCl.
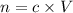
Where
c is molarity
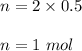
Let V be the volume of the solution. So,
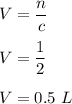
So, the required volume of the solution is equal to 0.5 L.

It will provide an instant answer!
