 9
9  9
9  6
6 Explanation:
The picture or the animation is missing. Therefore, it is not possible to represent the color of the bonds between the particles of NaC2H3O2.
However, The compound NaC2H3O2 is a sodium salt of acetic acid called Sodium acetate trihydrate. It is readily formed by the reaction of acetic acid (vinegar) and sodium hydroxide or the carbonates, bicarbonates of sodium.
 21
21 The temperature of the solute/solvent without any external effect would decrease.
Explanation:
As the bonding between the solute particles is really strong, therefore a large amount of energy is required to overcome these forces. So that the new bonding between the solute and solvent is created.
In order to achieve this, there will be a lot of energy required and that is through the heating process. So the solution will require energy so the solute will dissolve fully either by provision of external force i.e stirring or by heating.
 6
6 Explanation:
The picture or the animation is missing. Therefore, it is not possible to represent the color of the bonds between the particles of NaC2H3O2.
However, The compound NaC2H3O2 is a sodium salt of acetic acid called Sodium acetate trihydrate. It is readily formed by the reaction of acetic acid (vinegar) and sodium hydroxide or the carbonates, bicarbonates of sodium.
 21
21 The temperature of the solute/solvent without any external effect would decrease.
Explanation:
As the bonding between the solute particles is really strong, therefore a large amount of energy is required to overcome these forces. So that the new bonding between the solute and solvent is created.
In order to achieve this, there will be a lot of energy required and that is through the heating process. So the solution will require energy so the solute will dissolve fully either by provision of external force i.e stirring or by heating.
Explanation:
Intermolecular force s between particles in a matter holds particles of molecules together in substances.
The stronger the intermolecular forces the more solid a substance is. The weakest intermolecular attraction are found in gases as they have negligible attractive forces in between their particles. Solids are held together by very strong attractive forces. Liquids have weak forces holding them in place and this allows for a little movement. Gases are typically have very weak to negligible attractive forces. 18
18 The correct answer is: There must be potential energy in the bonds due to its particles position.
Explanation:
Potential energy is a kind of energy which is stored in an object due to its position or configuration. For example, if there is a ball in the fifth floor of a building, it has a determined potential energy which is converted to kinetic energy when the ball fall down. The same is in the case of the chemical bonds: the particles or atoms have potential energy related to their relative positions in the system. In fact, chemical energy is considered a form of potential energy (stored in chemical bonds).
 18
18 The correct answer is: There must be potential energy in the bonds due to its particles position.
Explanation:
Potential energy is a kind of energy which is stored in an object due to its position or configuration. For example, if there is a ball in the fifth floor of a building, it has a determined potential energy which is converted to kinetic energy when the ball fall down. The same is in the case of the chemical bonds: the particles or atoms have potential energy related to their relative positions in the system. In fact, chemical energy is considered a form of potential energy (stored in chemical bonds).
 5
5 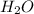 is an example.
is an example. 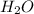 is made up of Hydrogen and Oxygen. Both of these elements are gases but when they come together they form a liquid.An ionic bond: molecule loses or gains an electron. A covalent bond: two molecules share an electron.
is made up of Hydrogen and Oxygen. Both of these elements are gases but when they come together they form a liquid.An ionic bond: molecule loses or gains an electron. A covalent bond: two molecules share an electron. Explanation:
For Goodness sakes figure the rest out on your ownI gave you a head start ;)
It will provide an instant answer!
