 8
8 1. Converting 11.03 moles of calcium nitrate to grams gives 1,809.89 grams.
2. The number of molecules that are contained in 103.4 g of sulfuric acid is 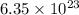 molecules.
molecules.
3. Converting  molecules of dinitrogen pentoxide to moles is equal to 5.3987 moles.
molecules of dinitrogen pentoxide to moles is equal to 5.3987 moles.
4. The mass of 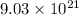 molecules of hydrobromic acid is 1.2135 grams.
molecules of hydrobromic acid is 1.2135 grams.
5. The number of moles contained in 26.29 g of iron (III) chloride is 0.1621 moles.
Given the following data:
Number of moles of calcium nitrate = 11.03 molesMass of sulfuric acid = 103.4 gramsNumber of molecules of dinitrogen pentoxide = Number of molecules of hydrobromic acid =
Number of molecules of hydrobromic acid = 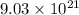 Mass of iron (III) chloride = 26.29 grams
Mass of iron (III) chloride = 26.29 gramsScientific data:
The molar mass of calcium nitrate = 46.07 g/mol.The molar mass of sulfuric acid = 98.079 g/mol.The molar mass of hydrobromic acid = 80.91 g/mol.The molar mass of iron (III) chloride = 162.2 g/mol.Avogadro's number =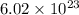
1. To convert 11.03 moles of calcium nitrate to grams:
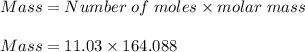
Mass of calcium nitrate = 1,809.89 grams.
2. To calculate how many molecules are contained in 103.4 g of sulfuric acid:
First of all, we would determine the number of moles contained in 103.4 g of sulfuric acid.
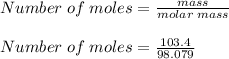
Number of moles = 1.0543 moles
By stoichiometry:
1 mole of sulfuric acid = 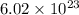 molecules
molecules
1.0543 mole of sulfuric acid = X molecules
Cross-multiplying, we have:
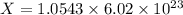
X = 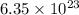 molecules
molecules
3. To convert  molecules of dinitrogen pentoxide to moles:
molecules of dinitrogen pentoxide to moles:
By stoichiometry:
1 mole of dinitrogen pentoxide = 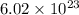 molecules
molecules
X mole of dinitrogen pentoxide =  molecules
molecules
Cross-multiplying, we have:
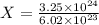
X = 5.3987 moles
4. To calculate the mass of 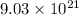 molecules of hydrobromic acid:
molecules of hydrobromic acid:
First of all, we would determine the number of moles contained in 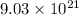 molecules of hydrobromic acid.
molecules of hydrobromic acid.
By stoichiometry:
1 mole of hydrobromic acid = 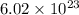 molecules
molecules
X mole of hydrobromic acid = 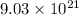 molecules
molecules
Cross-multiplying, we have:
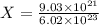
X = 0.015 moles
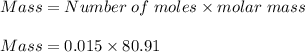
Mass of hydrobromic acid = 1.2135 grams.
5. To calculate the number of moles contained in 26.29 g of iron (III) chloride:
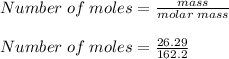
Number of moles = 0.1621 moles.
Read more: link
 8
8 1. Converting 11.03 moles of calcium nitrate to grams gives 1,809.89 grams.
2. The number of molecules that are contained in 103.4 g of sulfuric acid is 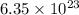 molecules.
molecules.
3. Converting  molecules of dinitrogen pentoxide to moles is equal to 5.3987 moles.
molecules of dinitrogen pentoxide to moles is equal to 5.3987 moles.
4. The mass of 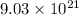 molecules of hydrobromic acid is 1.2135 grams.
molecules of hydrobromic acid is 1.2135 grams.
5. The number of moles contained in 26.29 g of iron (III) chloride is 0.1621 moles.
Given the following data:
Number of moles of calcium nitrate = 11.03 molesMass of sulfuric acid = 103.4 gramsNumber of molecules of dinitrogen pentoxide = Number of molecules of hydrobromic acid =
Number of molecules of hydrobromic acid = 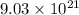 Mass of iron (III) chloride = 26.29 grams
Mass of iron (III) chloride = 26.29 gramsScientific data:
The molar mass of calcium nitrate = 46.07 g/mol.The molar mass of sulfuric acid = 98.079 g/mol.The molar mass of hydrobromic acid = 80.91 g/mol.The molar mass of iron (III) chloride = 162.2 g/mol.Avogadro's number =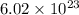
1. To convert 11.03 moles of calcium nitrate to grams:
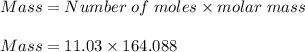
Mass of calcium nitrate = 1,809.89 grams.
2. To calculate how many molecules are contained in 103.4 g of sulfuric acid:
First of all, we would determine the number of moles contained in 103.4 g of sulfuric acid.
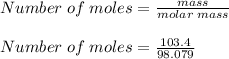
Number of moles = 1.0543 moles
By stoichiometry:
1 mole of sulfuric acid = 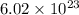 molecules
molecules
1.0543 mole of sulfuric acid = X molecules
Cross-multiplying, we have:
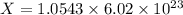
X = 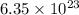 molecules
molecules
3. To convert  molecules of dinitrogen pentoxide to moles:
molecules of dinitrogen pentoxide to moles:
By stoichiometry:
1 mole of dinitrogen pentoxide = 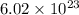 molecules
molecules
X mole of dinitrogen pentoxide =  molecules
molecules
Cross-multiplying, we have:
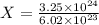
X = 5.3987 moles
4. To calculate the mass of 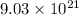 molecules of hydrobromic acid:
molecules of hydrobromic acid:
First of all, we would determine the number of moles contained in 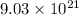 molecules of hydrobromic acid.
molecules of hydrobromic acid.
By stoichiometry:
1 mole of hydrobromic acid = 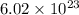 molecules
molecules
X mole of hydrobromic acid = 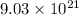 molecules
molecules
Cross-multiplying, we have:
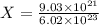
X = 0.015 moles
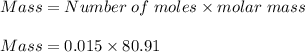
Mass of hydrobromic acid = 1.2135 grams.
5. To calculate the number of moles contained in 26.29 g of iron (III) chloride:
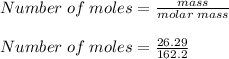
Number of moles = 0.1621 moles.
Read more: link
5.195 x 10²³ molecules ≅ 5.2 x 10²³ molecules.
Explanation:
It is known that every 1.0 mole of a molecule contains Avogadro's number of molecules (NA = 6.022 x 10²³).We need to calculate the no. of moles in 95.75 g of CaCl₂ using the relation:n = mass/molar mass = (95.75 g)/(110.98 g/mol) = 0.86 mol.
Using cross multiplication:
1.0 mole of CaCl₂ contains → 6.022 x 10²³ molecules.
0.86 mole of CaCl₂ contains → ??? molecules.
∴ 95.75 g of CaCl₂ (0.86 mole) contain = (0.86 mol)(6.022 x 10²³) = 5.195 x 10²³ molecules ≅ 5.2 x 10²³ molecules.
5.195 x 10²³ molecules ≅ 5.2 x 10²³ molecules.
Explanation:
It is known that every 1.0 mole of a molecule contains Avogadro's number of molecules (NA = 6.022 x 10²³).We need to calculate the no. of moles in 95.75 g of CaCl₂ using the relation:n = mass/molar mass = (95.75 g)/(110.98 g/mol) = 0.86 mol.
Using cross multiplication:
1.0 mole of CaCl₂ contains → 6.022 x 10²³ molecules.
0.86 mole of CaCl₂ contains → ??? molecules.
∴ 95.75 g of CaCl₂ (0.86 mole) contain = (0.86 mol)(6.022 x 10²³) = 5.195 x 10²³ molecules ≅ 5.2 x 10²³ molecules.
 4
4 For #1 The Answer is (A)Calcium gives an electron to chlorine to form a stable bond.
Hope that helps :D
 4
4 For #1 The Answer is (A)Calcium gives an electron to chlorine to form a stable bond.
Hope that helps :D
 1
1 Answer:
52.6 gramStep-by-step explanation:
It is clear by the equation 2(27+3×35.5)= 267 gm of AlCl3 reacts with 6× 80 = 480 gm of Br2 . So 29.2 gm reacts = 480× 29.2/267= 52.6 gm

It will provide an instant answer!
