 1
1 Weigh the empty crucible, and then weigh into it between 2 g and 3 g of hydrated copper(II) sulphate. Record all weighings accurate to the nearest 0.01 g.
Support the crucible securely in the pipe-clay triangle on the tripod over the Bunsen burner.
Heat the crucible and contents, gently at first, over a medium Bunsen flame, so that the water of crystallisation is driven off steadily. The blue colour of the hydrated compound should gradually fade to the greyish-white of anhydrous copper(II) sulfate. Avoid over-heating, which may cause further decomposition, and stop heating immediately if the colour starts to blacken. If over-heated, toxic or corrosive fumes may be evolved. A total heating time of about 10 minutes should be enough.
Allow the crucible and contents to cool. The tongs may be used to move the hot crucible from the hot pipe-clay triangle onto the heat resistant mat where it should cool more rapidly.
Re-weigh the crucible and contents once cold.
Calculation:
Calculate the molar masses of H2O and CuSO4 (Relative atomic masses: H=1, O=16, S=32, Cu=64)
Calculate the mass of water driven off, and the mass of anhydrous copper(II) sulfate formed in your experiment
Calculate the number of moles of anhydrous copper(II) sulfate formed
Calculate the number of moles of water driven off
Calculate how many moles of water would have been driven off if 1 mole of anhydrous copper(II) sulfate had been formed
Write down the formula for hydrated copper(II) sulfate.
#*#*SHOW FULLSCREEN*#*#
Explanation:
 1
1 Weigh the empty crucible, and then weigh into it between 2 g and 3 g of hydrated copper(II) sulphate. Record all weighings accurate to the nearest 0.01 g.
Support the crucible securely in the pipe-clay triangle on the tripod over the Bunsen burner.
Heat the crucible and contents, gently at first, over a medium Bunsen flame, so that the water of crystallisation is driven off steadily. The blue colour of the hydrated compound should gradually fade to the greyish-white of anhydrous copper(II) sulfate. Avoid over-heating, which may cause further decomposition, and stop heating immediately if the colour starts to blacken. If over-heated, toxic or corrosive fumes may be evolved. A total heating time of about 10 minutes should be enough.
Allow the crucible and contents to cool. The tongs may be used to move the hot crucible from the hot pipe-clay triangle onto the heat resistant mat where it should cool more rapidly.
Re-weigh the crucible and contents once cold.
Calculation:
Calculate the molar masses of H2O and CuSO4 (Relative atomic masses: H=1, O=16, S=32, Cu=64)
Calculate the mass of water driven off, and the mass of anhydrous copper(II) sulfate formed in your experiment
Calculate the number of moles of anhydrous copper(II) sulfate formed
Calculate the number of moles of water driven off
Calculate how many moles of water would have been driven off if 1 mole of anhydrous copper(II) sulfate had been formed
Write down the formula for hydrated copper(II) sulfate.
#*#*SHOW FULLSCREEN*#*#
Explanation:
Water of crystallization, X = 4.
Explanation:
Molar mass of 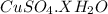
64 + 32 + (4x18) + x ( 1 × 2 + 16)
= 160 + 18x
Given: % water of crystallization (decrease in mass after heating) = 30%
⇒ 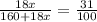
1800x = 31 (160 + 18x)
58.0645x = 160 + 18x
(58.0645 - 18)x = 160
x = 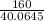 = 3.99 ≅ 4.
= 3.99 ≅ 4.
Water of crystallization, X = 4.
Water of crystallization, X = 4.
Explanation:
Molar mass of 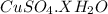
64 + 32 + (4x18) + x ( 1 × 2 + 16)
= 160 + 18x
Given: % water of crystallization (decrease in mass after heating) = 30%
⇒ 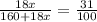
1800x = 31 (160 + 18x)
58.0645x = 160 + 18x
(58.0645 - 18)x = 160
x = 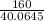 = 3.99 ≅ 4.
= 3.99 ≅ 4.
Water of crystallization, X = 4.
 5
5 The answer to your question is: CuSO₄ 5H₂O
Name: copper (II) sulfate pentahydrate
Explanation:
Data
copper (II) silfate hydrate = 3.97 g
copper (ii) sulfate mass = 2.54g
Correct formula = ?
CuSO₄ X H₂O
Process
Quantity of water = 3.97 - 2.54
= 1.43 g
MW of water
18 g of H2O 1 mol
1.43 g x
x = (1.43 x 1) / 18
x = 0.079 moles of water
MW CuSO₄ = 160 g
160 g 1 mol
2.54 g x
x = (2.54 x 1) / 160
x = 0.016 moles
Divide by the lowest number of moles
CuSO₂ = 0.016 / 0.016 = 1
H₂O = 0.079 / 0.016 = 4.9 ≈ 5
then, the formula will be
CuSO₄ 5H₂O
 5
5 The answer to your question is: CuSO₄ 5H₂O
Name: copper (II) sulfate pentahydrate
Explanation:
Data
copper (II) silfate hydrate = 3.97 g
copper (ii) sulfate mass = 2.54g
Correct formula = ?
CuSO₄ X H₂O
Process
Quantity of water = 3.97 - 2.54
= 1.43 g
MW of water
18 g of H2O 1 mol
1.43 g x
x = (1.43 x 1) / 18
x = 0.079 moles of water
MW CuSO₄ = 160 g
160 g 1 mol
2.54 g x
x = (2.54 x 1) / 160
x = 0.016 moles
Divide by the lowest number of moles
CuSO₂ = 0.016 / 0.016 = 1
H₂O = 0.079 / 0.016 = 4.9 ≈ 5
then, the formula will be
CuSO₄ 5H₂O
 2
2 36.0%.
Explanation:
The mass of water = mass of hydrated copper (II) sulfate - mass of copper (II) sulfate after heating = 4.56 g - 2.92 g = 1.64 g.
The percent by mass of water in the hydrate = (mass of water/mass of hydrated copper (II) sulfate)*100 = (1.64 g/4.56 g)*100 = 35.96% ≅ 36.0%.
 2
2 36.0%.
Explanation:
The mass of water = mass of hydrated copper (II) sulfate - mass of copper (II) sulfate after heating = 4.56 g - 2.92 g = 1.64 g.
The percent by mass of water in the hydrate = (mass of water/mass of hydrated copper (II) sulfate)*100 = (1.64 g/4.56 g)*100 = 35.96% ≅ 36.0%.
 164
164 The chemist should set up his or her stoichiometric calculation to predict the mass of CuSO4 that forms when a specified mass of CuSO4•5H2O is heated. By dividing first the mass of CuSO4•5H2O by its molar mass then multiply it by the stoichiometric ratio of CuSO4/ CuSO4•5H2O and then multiply the molar mass of CuSO4.
 164
164 The chemist should set up his or her stoichiometric calculation to predict the mass of CuSO4 that forms when a specified mass of CuSO4•5H2O is heated. By dividing first the mass of CuSO4•5H2O by its molar mass then multiply it by the stoichiometric ratio of CuSO4/ CuSO4•5H2O and then multiply the molar mass of CuSO4.

It will provide an instant answer!
