The molar mass of unknown diatomic gas is 38 grams
Explanation:
At STP:
The temperature and pressure conditions are 273 K and 1 atm respectively.
To calculate the number of moles, we use the equation given by ideal gas equation:
PV = nRT
P = Pressure of the gas = 1.00 atm
V = Volume of the gas = 12.88 L
n = number of moles of mixture = ?
R = Gas constant = 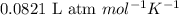
T = Temperature of the gas
Putting values in above equation, we get:
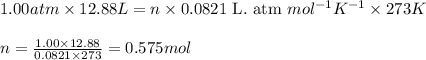
To calculate the number of moles, we use the equation:
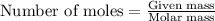 .....(1)
.....(1)
For Argon:
Given mass of argon = 5.7 g
Molar mass of argon = 39.95 g/mol
Putting values in equation 1, we get:
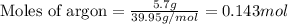
For neon:
Given mass of neon = 5.7 g
Molar mass of neon = 20.2 g/mol
Putting values in equation 1, we get:
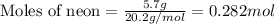
Moles of unknown diatomic gas = [0.575 - (0.143 + 0.282)] = 0.150
Now, calculating the molar mass of unknown diatomic gas from equation 1, we get:
Given mass of unknown diatomic gas = 5.7 g
Moles of unknown diatomic gas = 0.150 moles
Putting values in equation 1, we get:

Hence, the molar mass of unknown diatomic gas is 38 grams