Answer:
There are various variables matter to investigate the cell reaction like Voltage, Time etc.Step-by-step explanation:
For a Graphic organizer it matter to investigate the cell reaction on the basis of following variables Voltage, Time , heating effect of cell reaction, material of electrodes, electrolyte etc.
Answer:
There are a number of variables like Voltage, Back up time, electrodes, electrolytes and heating effect of battery etc.Step-by-step explanation:
For a graphic organizer it is very important to know the voltage provided by the cell, time back up of cell reaction, electrodes and electrolyte involved in celll reaction. It is very important that the cell reaction must have minimum heating effect.
Answer:
AStep-by-step explanation:
The input force is 50 N. But it will not create not any change. No mechanical advantage is observed.
 1
1 Answer:
52.6 gramStep-by-step explanation:
It is clear by the equation 2(27+3×35.5)= 267 gm of AlCl3 reacts with 6× 80 = 480 gm of Br2 . So 29.2 gm reacts = 480× 29.2/267= 52.6 gm
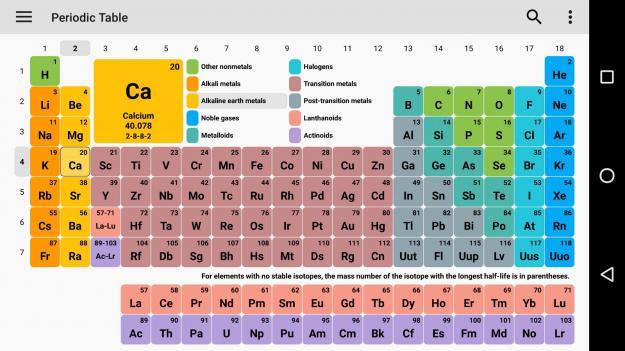
Calcium (Ca)(On the periodic table, ionization energy increases as you go up and to the right of the periodic table)

It will provide an instant answer!
