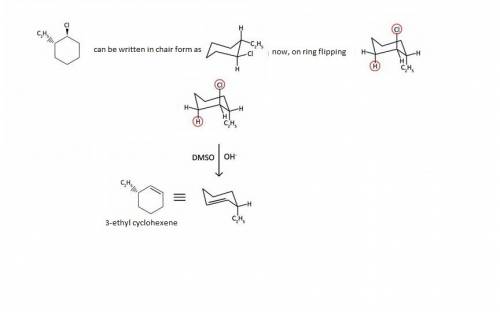
Solution: E2 reaction is one of the two types of elimination reactions in which removal of two substituents take place from a single molecule. E2 reaction is a one-step reaction.
When hydroxide ion OH^{-} reacts with trans-1-chloro-2-ethylcyclohexane, OH^{-} will act as a nucleophile and starts the elimination reaction.
Here, trans-1-chloro-2-ethylcyclohexane means chlorine and ethyl groups are trans to each other. The hydroxide ion will attack on the hydrogen atom, anti per planar to chlorine substituent thus, hydrogen and chlorine eliminate in the single step to form stable alkene. The major product so formed will be 3-ethyl cyclohexene.
The reaction is in the attachment.