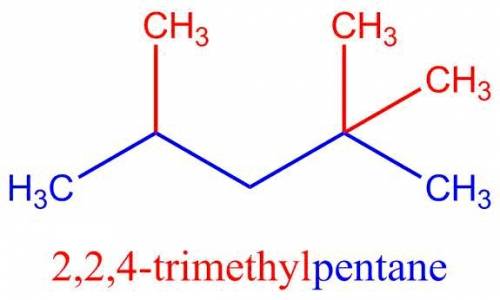
The structure of Isooctane is shown below,
IUPAC Name:
IUPAC name is assigned according to following rules.
1) Select the longest Chain:
A longest chain of five carbon atoms was selected. The longest carbon chain is highlighted with Blue color. As there are five carbons in longest chain, so the parent name for this compound is Pentane.
2) Substituent Numbering:
There are two options to start numbering either from one or another end. According to rules numbering should be started from the end close to many substituents. So, carbon 1 was assigned nearer to two methyl groups.
There are three methyl substituents, two at position 2 and one at position 4 (Highlighted RED). Hence, 2,2,4-Trimethyl.
Result:
So, the final name is,
2,2,4-Trimethylpentane