Feed steam is 1100 kg/h
20% of Ethanol (220 kg/h)
36.36% of propanol (400 Kg/h)
43.64% of methanol (480 Kg/h)
Distilled steam is 542 Kg/h
20.30 % of etanol (110 kg/h)
79.70 % of methanol (432 kg/h)
Bottom steam is 558 Kg/h
19.71% of Ethanol (110 kg/h)
8.60% of propanol (400Kg/h)
71.68% of methanol (48 Kg/h)
Explanation:
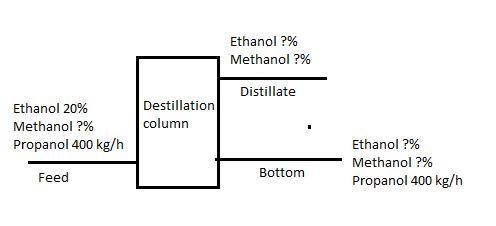
The attached figure shows the distillation scheme representation. There are three steams, feed, distillate and Bottom, the feed and Bottom steam have ethanol, methanol and propanol, and the distillate steam only has ethanol and methanol.
Solving Feed Steam:
The problem says that 20% of the feed is ethanol:
Ethanol feed= (1100 Kg/h)*(0.20)= 220 Kg/h
The problema also says that propanol feed is 400 kg/h, so the sum of ethanol+methanol+propanol=1100
Methanol= (1100- 400-220)kg/h= 480 Kg/h
Solving Distillate steam
The problem says that 90% of the feed methanol was recovered in distillate steam whit the 50% of the feed ethanol
Methanol= (480 Kg/h)*(0.9)=432 Kg/h
Ethanol= (220 Kg/h)*(0.5)= 110 Kg/h
Solving Bottom steam
If the 90% of the feed methanol and the 50% of the feed ethanol were recovered in distillate steam, so 10% and 50% were recovered in Bottom steam, respectively. Also, the problem says all the propanol was recovered in Bottom steam
Methanol= (480 Kg/h)*(0.1)=48 Kg/h
Ethanol= (220 Kg/h)*(0.5)= 110 Kg/h
Propanol= 400 Kg/h