Octane will be used completely.
Explanation:
To calculate the moles :
The balanced chemical reaction will be
According to stoichiometry :
2 moles of octane require = 25 moles of 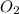
Thus 0.0202 moles of octane will require=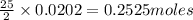 of
of 
Thus octane is the limiting reagent as it limits the formation of product and 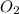 is the excess reagent.
is the excess reagent.
Thus octane will be used completely.