Answer
Answer 1 : 28.9 g of CO is needed.
Answer 2 : Six moles of  over Nine moles of
over Nine moles of 
Answer 3 : Four over two fraction can be used for the mole ratio to determine the mass of Fe from a known mass of  .
.
Answer 4 : Mass of  = (150 × 3 × 31.998) ÷ (232.29 × 1) grams
= (150 × 3 × 31.998) ÷ (232.29 × 1) grams
Answer 5 : 8.4 moles of sodium cyanide (NaCN) would be needed.
Solution
Solution 1 : Given,
Given mass of  = 55 g
= 55 g
Molar mass of  = 159.69 g/mole
= 159.69 g/mole
Molar mass of CO = 28.01 g/mole
Moles of  =
=  =
=  = 0.344 moles
= 0.344 moles
Balanced chemical reaction is,
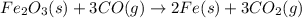
From the given reaction, we conclude that
1 mole of  gives → 3 moles of CO
gives → 3 moles of CO
0.344 moles of  gives → 3 × 0.344 moles of CO
gives → 3 × 0.344 moles of CO
= 1.032 moles
Mass of CO = Number of moles of CO × Molar mass of CO
= 1.032 × 28.01
= 28.90 g
Solution 2 : The balanced chemical reaction is,

From the given reaction, we conclude that the Six moles of  over Nine moles of
over Nine moles of  is the correct option.
is the correct option.
Solution 3 : The balanced chemical reaction is,

From the given balanced reaction, we conclude that Four over two fraction can be used for the mole ratio to determine the mass of Fe from a known mass of  .
.
Solution 4 : Given,
Given mass of  = 150 g
= 150 g
Molar mass of  = 232.29 g/mole
= 232.29 g/mole
Molar mass of  = 31.998 g/mole
= 31.998 g/mole
Moles of  =
=  =
= 
The balanced chemical equation is,

From the given balanced equation, we conclude that
1 mole of  gives → 3 moles of
gives → 3 moles of 
 of
of  gives →
gives → ![[(\frac{150\times 1}{232.29})\times 3] moles](/tpl/images/0390/3270/cd451.png) of
of 
Mass of  = Number of moles of
= Number of moles of  × Molar mass of
× Molar mass of  =
= ![[(\frac{150\times 1}{232.29})\times 3] \times 31.998 grams](/tpl/images/0390/3270/9e58a.png)
Therefore, the mass of  = (150 × 3 × 31.998) ÷ (232.29 × 1) grams
= (150 × 3 × 31.998) ÷ (232.29 × 1) grams
Solution 5 : Given,
Number of moles of  = 4.2 moles
= 4.2 moles
Balanced chemical equation is,

From the given chemical reaction, we conclude that
1 mole of  obtained from 2 moles of NaCN
obtained from 2 moles of NaCN
4.2 moles of  obtained → 2 × 4.2 moles of NaCN
obtained → 2 × 4.2 moles of NaCN
Therefore,
The moles of NaCN needed = 2 × 4.2 = 8.4 moles