1. The solubility of the anesthetic gas 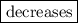 if the temperature is increased.
if the temperature is increased.
2. The solubility of the anesthetic gas 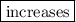 if its partial pressure is increased.
if its partial pressure is increased.
3. The solubility of the oxygen 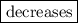 if the temperature of the body is increased.
if the temperature of the body is increased.
4. The solubility of oxygen 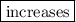 if its pressure over the solvent is increased.
if its pressure over the solvent is increased.
5. The solubility of air in blood 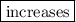 if diver descends
if diver descends 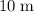 and the pressure is increased by
and the pressure is increased by 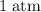 .
.
Further Explanation:
The chemical property of any substance that makes it capable to dissolve in other substances is called solubility. It is measured in terms of the maximum amount of solute that can be dissolved in the given amount of solvent.
Solubility is directly related to the intermolecular forces. Moreover, temperature and pressure are two other factors that influence the solubility of the gas.
There exist weak intermolecular forces between the gas molecules. With the increase in the temperature, the average kinetic energy also speeds up due to which the molecules start vibrating about their mean positions. This results in overcoming of the weak forces and the molecules re-enter into the gas phase. So solubility decreases with the increase in temperature.
The law that governs the relationship of gas solubility with its partial pressure is Henry’s Law. It states that the solubility 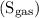 is directly proportional to the partial pressure
is directly proportional to the partial pressure 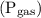 of the gas. If the gas has high partial pressure, it will have a higher solubility and vice-versa.
of the gas. If the gas has high partial pressure, it will have a higher solubility and vice-versa.
Mathematically, 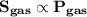
We incorporate a proportionality constant in order to remove the sign of proportionality. That constant is known as Henry’s constant, represented by 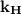 .
.
So Henry’s Law becomes
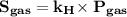
1. As we know, the solubility of gas is inversely proportional to the temperature. So if the temperature of anesthetic gas is increased, its solubility decreases.
2. According to Henry’s Law, the solubility of gas is directly proportional to the partial pressure of the gas. So the solubility of anesthetic gas also increases with the increase in its partial pressure.
3. As we know, the solubility of gas is inversely proportional to the temperature. So if the temperature of the body is increased, the solubility of oxygen decreases.
4. According to Henry’s Law, the solubility of gas is directly proportional to the partial pressure of the gas. So the solubility of oxygen also increases with the increase in its partial pressure.
5. As the diver descends 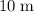 and the pressure is increased by
and the pressure is increased by 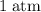 , the solubility of gas increases in accordance with Henry’s Law.
, the solubility of gas increases in accordance with Henry’s Law.
Learn more:
1. Statement about subatomic particle:
2. The energy of a photon in light:
Answer details:
Grade: Senior School
Subject: Chemistry
Chapter: Solutions
Keywords: solubility, gas, increase, decrease, temperature, partial pressure, diver, air, change, environment, oxygen, blood, Henry's Law, anesthetic gas.