Explanation:
It is known that 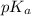 of propionic acid = 4.87
of propionic acid = 4.87
And, initial concentration of propionic acid = 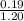
= 0.158 M
Concentration of sodium propionate = \frac{0.26}{1.20}[/tex]
= 0.216 M
Now, in the given situation only propionic acid and sodium propionate are present
.
Hence, pH = ![pK_{a} + log(\frac{[salt]}{[acid]})](/tpl/images/0350/6880/fe481.png)
= 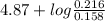
= 4.87 + log (1.36)
= 5.00
Therefore, when 0.02 mol NaOH is added then,
Moles of propionic acid = 0.19 - 0.02
= 0.17 mol
Hence, concentration of propionic acid = 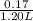
= 0.14 M
and, moles of sodium propionic acid = (0.26 + 0.02) mol
= 0.28 mol
Hence, concentration of sodium propionic acid will be calculated as follows.
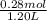
= 0.23 M
Therefore, calculate the pH upon addition of 0.02 mol of NaOH as follows.
pH = ![pK_{a} + log(\frac{[salt]}{[acid]})](/tpl/images/0350/6880/fe481.png)
= 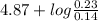
= 4.87 + log (1.64)
= 5.08
Hence, the pH of the buffer after the addition of 0.02 mol of NaOH is 5.08.
Therefore, when 0.02 mol HI is added then,
Moles of propionic acid = 0.19 + 0.02
= 0.21 mol
Hence, concentration of propionic acid = 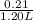
= 0.175 M
and, moles of sodium propionic acid = (0.26 - 0.02) mol
= 0.24 mol
Hence, concentration of sodium propionic acid will be calculated as follows.
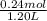
= 0.2 M
Therefore, calculate pH upon addition of 0.02 mol of HI as follows.
pH = ![pK_{a} + log(\frac{[salt]}{[acid]})](/tpl/images/0350/6880/fe481.png)
= 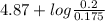
= 4.87 + log (0.114)
= 4.98
Hence, the pH of the buffer after the addition of 0.02 mol of HI is 4.98.