 9
9 a)135.9 grams
b) 10 grams
c) 8.83g
d) 88.3g
Explanation:
the balanced reaction shows that four molecules of iron (II) dichromate will react with eight molecules of potassium carbonate and one molecule of oxygen to give two molecules of ferric oxide, eight molecules of potassium chormate and carbon dioxide each.
a) How many grams of iron (II) dichromate are required to produce 44.0 grams of carbon dioxide?
The molar mass of carbon dioxide = 44 g/mol
The molar mass of iron (II) dichromate = 271.8 g/mol
Thus as we need one mole of carbon dioxide we need to have half moles of iron (II) dichromate = 0.5 X 271.8 = 135.9 grams
b) How many grams of oxygen gas are required to produce 100.0 grams of ferric oxide?
The molar mass of ferric oxide =160 g/mol
the molar mass of oxygen = 32 g/mol
two moles of ferric oxide are obtained from one mole of oxygen
thus
320g of ferric oxide from 32 grams of oxygen
for 100g of ferric oxide the mass of oxygen required = 10 grams
c) If 300.0 grams of iron (II) dichromate react, how many grams of oxygen gas will be consumed?
The molar mass of iron (II) dichromate = 271.8 g/mol
the molar mass of oxygen = 32 g/mol
With four moles of iron (II) dichromate one mole of oxygen reacts
thus 4 X 271.8 grams of iron (II) dichromate reacts with = 32g of oxygen
hence 300 grams will react with =
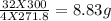
d) How many grams of iron (III) oxide will be produced from 300.0 grams of ferrous dichromate?
The molar mass of iron (II) dichromate = 271.8 g/mol
The molar mass of ferric oxide =160 g/mol
from 4 moles of iron dichromate two moles of ferric oxides are formed
thus
4 X 271.8 grams of iron dichromate will give = 2X160 g of ferric oxide
300 grams will give =
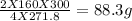
 9
9 a)135.9 grams
b) 10 grams
c) 8.83g
d) 88.3g
Explanation:
the balanced reaction shows that four molecules of iron (II) dichromate will react with eight molecules of potassium carbonate and one molecule of oxygen to give two molecules of ferric oxide, eight molecules of potassium chormate and carbon dioxide each.
a) How many grams of iron (II) dichromate are required to produce 44.0 grams of carbon dioxide?
The molar mass of carbon dioxide = 44 g/mol
The molar mass of iron (II) dichromate = 271.8 g/mol
Thus as we need one mole of carbon dioxide we need to have half moles of iron (II) dichromate = 0.5 X 271.8 = 135.9 grams
b) How many grams of oxygen gas are required to produce 100.0 grams of ferric oxide?
The molar mass of ferric oxide =160 g/mol
the molar mass of oxygen = 32 g/mol
two moles of ferric oxide are obtained from one mole of oxygen
thus
320g of ferric oxide from 32 grams of oxygen
for 100g of ferric oxide the mass of oxygen required = 10 grams
c) If 300.0 grams of iron (II) dichromate react, how many grams of oxygen gas will be consumed?
The molar mass of iron (II) dichromate = 271.8 g/mol
the molar mass of oxygen = 32 g/mol
With four moles of iron (II) dichromate one mole of oxygen reacts
thus 4 X 271.8 grams of iron (II) dichromate reacts with = 32g of oxygen
hence 300 grams will react with =
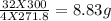
d) How many grams of iron (III) oxide will be produced from 300.0 grams of ferrous dichromate?
The molar mass of iron (II) dichromate = 271.8 g/mol
The molar mass of ferric oxide =160 g/mol
from 4 moles of iron dichromate two moles of ferric oxides are formed
thus
4 X 271.8 grams of iron dichromate will give = 2X160 g of ferric oxide
300 grams will give =
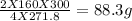
 3
3 12.6g of Fe.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is given below:
2Fe2O3(s) —> 4Fe(s) + 3O2(g)
Next, we shall determine the mass of Fe2O3 that decomposed and the mass of Fe that is produced from the balanced equation.
This is illustrated below:
Mola mass of Fe2O3 = (56 x 2) + (16x3) = 160g/mol
Mass of Fe2O3 from the balanced equation = 2 x 160= 320g
Molar mass of Fe = 56g/mol
Mass of Fe from the balanced equation = 4 x 56 = 224g
From the balanced equation above,
320g of Fe2O3 decomposes to produce 224g of Fe.
Finall, we can obtain the mass of the Fe produced from the decomposition of 18g of Fe2O3 as follow:
From the balanced equation above,
320g of Fe2O3 decomposes to produce 224g of Fe.
Therefore 18g of Fe2O3 will decompose to produce = (18x224)/320 = 12.6g of Fe.
Therefore, 12.6g of Fe is obtained from 18g of Fe2O3.
 3
3 12.6g of Fe.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is given below:
2Fe2O3(s) —> 4Fe(s) + 3O2(g)
Next, we shall determine the mass of Fe2O3 that decomposed and the mass of Fe that is produced from the balanced equation.
This is illustrated below:
Mola mass of Fe2O3 = (56 x 2) + (16x3) = 160g/mol
Mass of Fe2O3 from the balanced equation = 2 x 160= 320g
Molar mass of Fe = 56g/mol
Mass of Fe from the balanced equation = 4 x 56 = 224g
From the balanced equation above,
320g of Fe2O3 decomposes to produce 224g of Fe.
Finall, we can obtain the mass of the Fe produced from the decomposition of 18g of Fe2O3 as follow:
From the balanced equation above,
320g of Fe2O3 decomposes to produce 224g of Fe.
Therefore 18g of Fe2O3 will decompose to produce = (18x224)/320 = 12.6g of Fe.
Therefore, 12.6g of Fe is obtained from 18g of Fe2O3.
 2
2 im stuck on it too unfortunatley
Explanation:
 2
2 im stuck on it too unfortunatley
Explanation:
 11
11 Answer : The correct option is, (b) 22.1 g
Solution : Given,
Mass of iron = 15.5 g
Molar mass of iron = 56 g/mole
Molar mass of 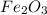 = 160 g/mole
= 160 g/mole
First we have to calculate the moles of iron.
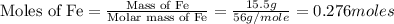
Now we have to calculate the moles of 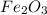 .
.
The balanced reaction is,
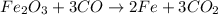
From the balanced reaction, we conclude that
As, 2 moles of iron obtained from 1 mole of 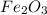
So, 0.276 moles of iron obtained from 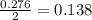 mole of
mole of 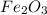
Now we have to calculate the mass of 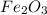
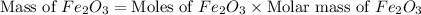
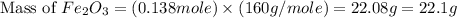
Therefore, the amount of 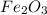 required are, 22.1 grams.
required are, 22.1 grams.
 11
11 Answer : The correct option is, (b) 22.1 g
Solution : Given,
Mass of iron = 15.5 g
Molar mass of iron = 56 g/mole
Molar mass of 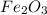 = 160 g/mole
= 160 g/mole
First we have to calculate the moles of iron.
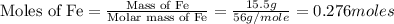
Now we have to calculate the moles of 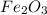 .
.
The balanced reaction is,
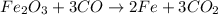
From the balanced reaction, we conclude that
As, 2 moles of iron obtained from 1 mole of 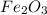
So, 0.276 moles of iron obtained from 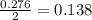 mole of
mole of 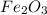
Now we have to calculate the mass of 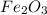
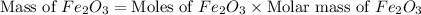
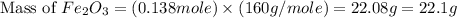
Therefore, the amount of 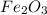 required are, 22.1 grams.
required are, 22.1 grams.
1.04g of NaOH must be added to precipitate all of the iron
Explanation:
Based on the chemical reaction:
Fe(NO₃)₂(aq) + 2NaOH(aq) → Fe(OH)₂(s) + 2NaNO₃(aq)
Where 1 mole of Iron (II) nitrate reacts with 2 moles of NaOH to produce 1 mole of Fe(OH)₂
To solve this question we need to find the moles of Fe(NO₃)₂ and using the chemical reaction we can find the moles of NaOH and its mass:
Moles Fe(NO₃)₂:
0.0427L * (0.305mol / L) = 0.0130moles of Fe(NO₃)₂
Moles NaOH
0.0130moles of Fe(NO₃)₂ * (2mol NaOH / 1mol Fe(NO₃)₂) =
0.0260 moles NaOH
Mass -Molar mass NaOH = 40g/mol-:
0.0260 moles NaOH * (40g / mol) =
1.04g of NaOH must be added to precipitate all of the iron 2
2 1. D (24.0 moles CO2)
2. A (.239 moles H2)
Explanation:
1. First Balance the equation
1 C3H8 + 5 O2 ---> 3 CO2 + 4 H2O
Then set up a stoiciometric equation so that the moles of O2 cancel out
40mol O2 x 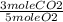 = 24.0 moles CO2
= 24.0 moles CO2
2. Set up a stoichiometric equation
10 grams Fe x 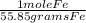 x
x 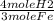 = 0.239 moles H2
= 0.239 moles H2

It will provide an instant answer!
