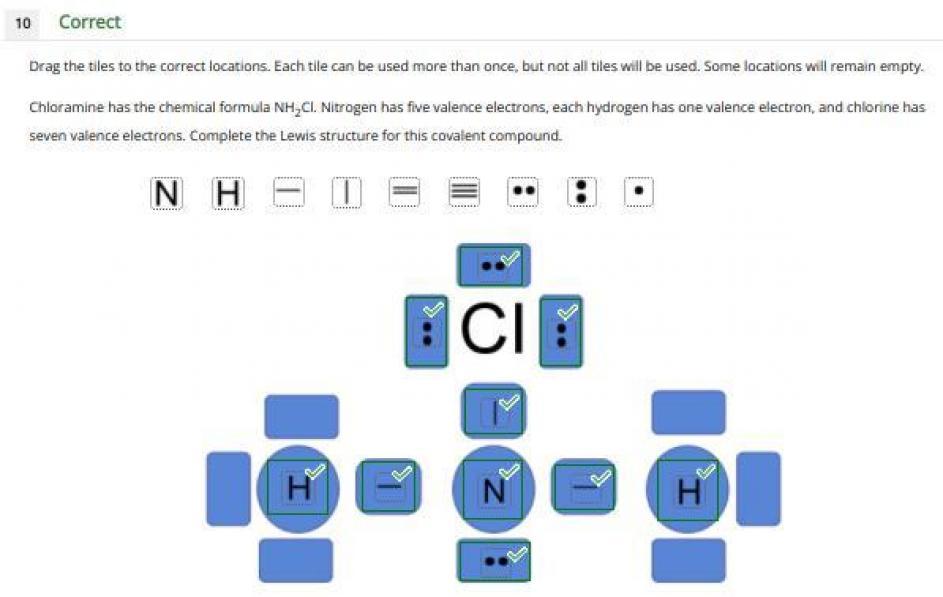
Answer:
A lewis structure shows the electrons on the valence shell of a molecule as dots.
Explanation:
A lewis structure refers to a structure in which dots are used to represent the electrons present on the outermost shells of the atoms comprising the bond. So stones a line is used to show a pair of electrons shared by two atoms in a covalent bond.
This system was put toward by Sir G.N Lewis in 1916 in his article titled, 'The atom and the molecule'. Lewis structures are based on the octet rule.
The image attached shows the lewis structure of chloramine. All the electrons present on the valence shell of all the bonding atoms is adequately shown in the image. There are fourteen valence electrons in the molecule as shown in the diagram. Recall that chlorine has seven valence electrons, nitrogen has five and each hydrogen atom has one valence electron each. The three lone pairs on the chlorine atom and one lone pair on the nitrogen atom is clearly shown in the image.