Options:
A.The spectrum shows a single electron in the 2p subshell.
B.The spectrum shows equal numbers of electrons in the first and second electron shells.
C.The spectrum shows three electrons with the same binding energy in the second electron shell.
Answer:
A. The spectrum shows a single electron in the 2p subshell.
Explanation:
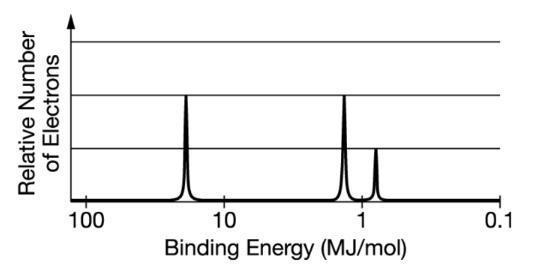
The figure attached is representation of the photoelectron spectrum for the element boron, which is the missing part of the question.
There are three peaks. Each peak represents one subshell: 1s, 2s, and 2p.
Indeed, Boron has 5 electrons: 2 in the subshell 1s, 2 in the subshell 2s and one in the subshell 2p.
The first peak, the peak to the left of the diagram, is as high as the second peak, meaining there are the same relative number of electrons in the 1s and 2s subshells.
The third peak, the one to the right of the diagram is half as high as the other two peaks, there are half electrons in the 2p subshell as in the 1s and 2s subshells.
Since Boron has five electrons, the photoelectrum spectrum shows that there are two electrons in the 1s subshell, two electrons in the 2s subshell, and a single electron in the 2p subshell.
Then, as for the answer choices:
A The spectrum shows a single electron in the 2p subshell: correct as explained in the last part of the previous paragraph.
B The spectrum shows equal numbers of electrons in the first and second electron shells: incorrect as it shows two electrons in the first shell (1s) and three electrons in the second shell (2s and 2p).
C The spectrum shows three electrons with the same binding energy in the second electron shell: incorrect as it shows different binding energies for the electrons in the second electron shell: two electrons below 1 MJ/mol, and one above 1 MJ/mol.