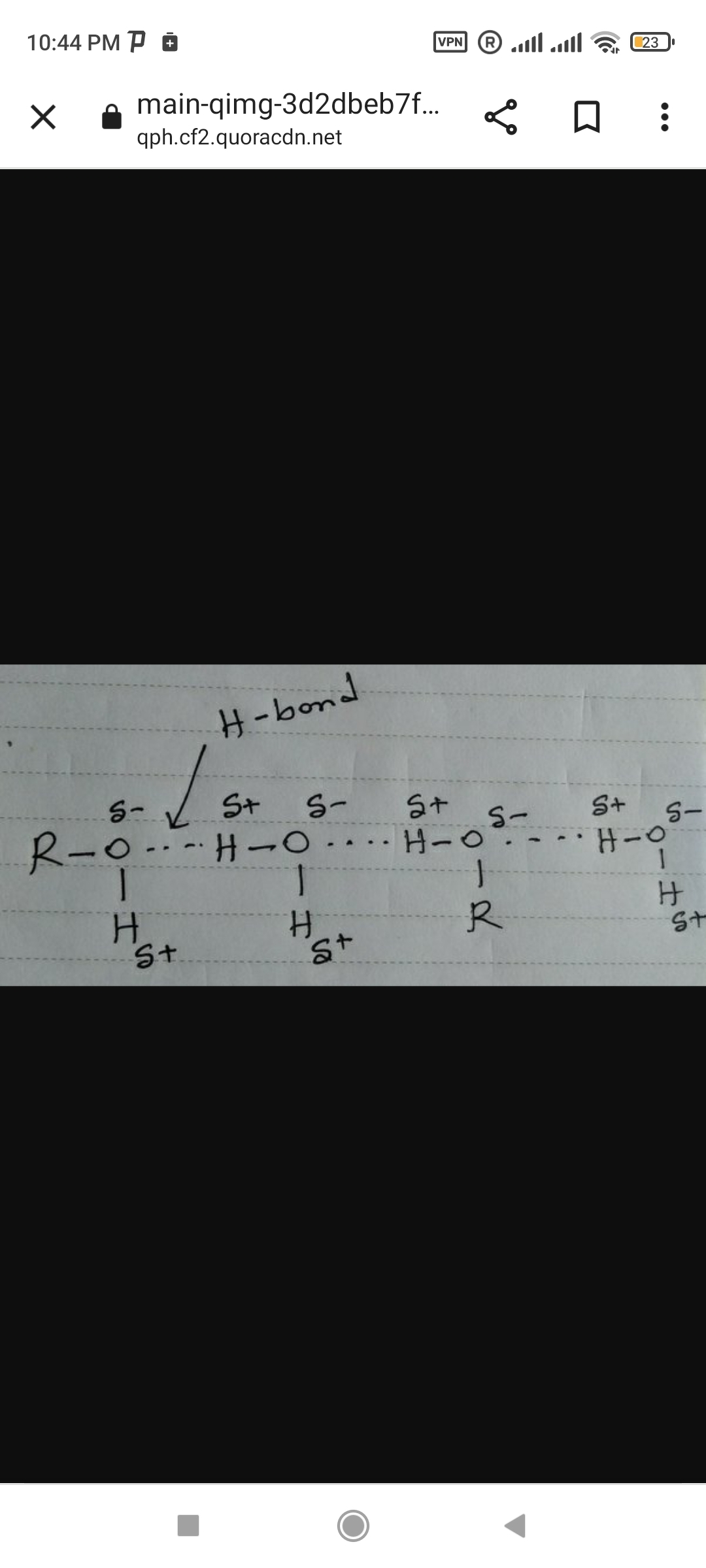
Answer:
Solubility of alcohol in waterStep-by-step explanation:
The general formula of alcohol is R-OH. In hydroxyl group of alcohol, the electronegativity values of oxygen and hydrogen are 3.5 & 2.1 respectively i.e the electronegativity difference is 1.4.
Due to high electronegativity difference oxygen gained partial negative charge and hydrogen gained partial positive charge.
Hence, the negative pole of alcohol is attracted by positive pole of water and the positive pole of alcohol is attracted by negative pole of water due to which alcohol can form hydrogen bond with water. Therefore, alcohol soluble in water.