Q1:
M = 1.0 mol/L.
Explanation:
•To solve this problem, we can use the relation:
M = 10Pd / Molar mass, where
M is the molarity of the solution (mol/L),
P is the percent of the solution (P = 7.06 %),
d is the density of the solution (d = 1.19 g/ml),
Molar mass of sodium bicarbonate (84.007 g/mol),
M = 10 (7.06 %) (1.19 g/ml) / (84.007 g/mol) = 1.0 mol/L.
Q2:
Molality = 0.904 m.
Explanation:
•To solve this problem, we can use the relation of molality (m):
m = (mass / molar mass) solute x (1000 / mass of solvent)
The solute is sodium bicarbonate (7.06 %), it is the component with the lower percent.
The solvent is water (92.94 %), it is the component with the higher percent.
•To get the mass of the solute and solvent, we should firstly get the mass of the solution.
Let assume that the solution has a volume of 1.0 L (1000 ml).
Then the mass of the solution = d x V = (1.19 g/ml) (1000 ml) = 1190 g.
The mass of solute sodium bicarbonate = (the total mass of the solution x percent % of solute) / 100 = (1190 x 7.06) / 100 = 84.014 g.
The mass of solvent water = (the total mass of the solution x percent % of solvent) / 100 = (1190 x 92.94) / 100 = 1105.986 g.
m = (mass / molar mass) solute x (1000 / mass of solvent)
m = (84.014 g / 84.007 g/mol) (1000 / 1105.986 g) = 0.904 m.
Q3:
Mole fraction of solvent (water) = 0.953.
Explanation:
•The solute is methanol CH3OH (8.05 %), it is the component with the lower percent.
•The solvent is water (91.95 %), it is the component with the higher percent.
•To solve this problem, we can use the relation of mole fraction of water (X water):
X water = (no. of moles of water) / (total no. of moles of the solution)
•To get the no. of moles of the solute and solvent, we should obtain the mass of both.
•To obtain the mass of the solute and solvent, we should firstly get the mass of the solution.
•Let assume that the solution has a volume of 1.0 L (1000 ml).
Then the mass of the solution = d x V = (0.976 g/ml) (1000 ml) = 976.0 g.
The mass of solute methanol CH3OH = (the total mass of the solution x percent % of solute) / 100 = (976.0 x 8.05) / 100 = 78.568 g.
The mass of solvent water = (the total mass of the solution x percent % of solvent) / 100 = (976.0 x 91.95) / 100 = 897.432 g.
The no. of moles of solute methanol CH3OH = mass / molar mass = (78.568 g) / (32.0 g/mol) = 2.45525 mol.
The no. of moles of solvent water = mass / molar mass = (897.432 g) / (18.0 g/mol) = 49.857 mol.
X water = (no. of moles of water) / (total no. of moles of the solution) = (49.857) / (49.857 + 2.45525) = 0.953.
Q4:
m = 5.18 m.
Explanation:
•To solve this problem, we can use the relation of molality (m):
m = (mass / molar mass) solute x (1000 / mass of solvent)
Mass of the solute cesium chloride = 52.3 g.
Molar mass of cesium chloride = 168.36 g/mol.
Mass of the solvent water = 60.0 g.
m = (mass / molar mass) cesium chloride x (1000 / mass of water)
m = (52.3 g / 168.36 g/mol) (1000 / 60.0 g) = 5.177 m = 5.18 m.
Q5:
4.91 M.
Explanation:
•To solve this problem, we can use the relation of molarity (M):
M = (mass / molar mass) solute x (1000 / V of the solution)
Mass of the solute cesium chloride = 52.3 g.
Molar mass of cesium chloride = 168.36 g/mol.
V of the solution = 63.3 ml.
M = (mass / molar mass) cesium chloride x (1000 / V of the solution)
M = (52.3 g / 168.36 g/mol) (1000 / 63.3 g) = 4.907 M = 4.91 M.
Q6:
The vapor pressure of the solution = 80.0 torr.
Explanation:
•The vapor pressure of the solution will be the partial vapor pressure of the ethanol only because alpha naphthol is assumed to be nonvolatile, so it has no partial vapor pressure in the total vapor pressure of the solution.
The vapor pressure of the solution = the partial vapor pressure of ethanol = (X ethanol) (Po ethanol),
Where, X ethanol is the mole fraction of ethanol.
P0 ethanol is the vapor pressure of pure ethanol (P0 = 100.0 torr).
•To get the mole fraction of ethanol, we can use the relation of mole fraction of ethanol (X ethanol):
X ethanol = (no. of moles of ethanol) / (total no. of moles of the solution).
•The no. of moles of ethanol = mass / molar mass = (36.8 g) / (46.07 g/mol) = 0.80 mol.
•The no. of moles of alpha naphthol = mass / molar mass = (28.8 g) / (144.0 g/mol) = 0.2 mol.
•X ethanol = (no. of moles of ethanol) / (total no. of moles of the solution) = (0.80) / (0.80 + 0.2) = 0.80.
The vapor pressure of the solution = the partial vapor pressure of ethanol = (X ethanol) (Po ethanol) = (0.80) (100.0 torr) = 80.0 torr.
Kindly find the answers of all questions in details attached as a word and pdf file due to the limitations of the capacity of the answer.
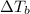 is the change in the water's boiling point (normally taken to be 100 °C),
is the change in the water's boiling point (normally taken to be 100 °C),  is the Van 't Hoff factor (the number of particles a single formula unit of the solute dissociates into in water),
is the Van 't Hoff factor (the number of particles a single formula unit of the solute dissociates into in water), 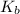 is the boiling point elevation constant, and
is the boiling point elevation constant, and 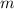 is the molality (moles of solute/kilogram(s) of solvent) of the solution.
is the molality (moles of solute/kilogram(s) of solvent) of the solution. , will be 2.
, will be 2.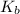 .
.