The boiling point of water increases as the amount of impurities dissolved in it increases. For our purposes, we will consider the non-electrolyte to be the dissolved impurity. The change in the boiling point can be calculated using the equation:
where 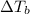 is the change in boiling point,
is the change in boiling point,  is the van ‘t Hoff factor (whose value denotes the number of particles each formula unit of the dissolved substance dissociates into in water),
is the van ‘t Hoff factor (whose value denotes the number of particles each formula unit of the dissolved substance dissociates into in water), 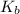 is the boiling point elevation constant, and
is the boiling point elevation constant, and 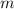 is the molality (moles of solute/kilogram of solvent) of the solution.
is the molality (moles of solute/kilogram of solvent) of the solution.
Right off the bat, since we're dealing with a non-electrolyte, the dissolved substance can be assumed not to dissociate in water. So, our van ‘t Hoff factor,  , would be 1 (by contrast, the
, would be 1 (by contrast, the  for an ionic compound like NaCl would be 2 since, in water, NaCl would dissociate into two particles: one Na⁺ ion and one Cl⁻ ion). We're also given our
for an ionic compound like NaCl would be 2 since, in water, NaCl would dissociate into two particles: one Na⁺ ion and one Cl⁻ ion). We're also given our 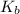 , which is 0.51 °C/m.
, which is 0.51 °C/m.
Assuming the normal boiling point of pure water to be 100 °C (a defined value for sig fig purposes), the change in boiling point from having dissolved 35.0 g of the non-electrolyte can be obtained by subtracting 100 °C from the final—elevated—boiling point of 101.25 °C:
Now, recall what we're asked to determine: the molecular mass of the dissolved substance. There is one unknown left in the equation: the molality of the solution. Let's first solve for that:
Notice that we didn't include the i since its value is 1.
Now, what would happen if we multiplied our molality by the mass of water we've been given? We would be left with the moles of solute. And what are we asked to find? The molecular mass, or the mass per mole. We can accomplish this in two steps. Remember to convert your mass of water to kilograms:
And, finally, we divide the mass of our solute by the number of moles of solute:
Our answer to two significant figures (which is the number of sig figs to which our 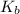 is given) would be 320 g/mol.
is given) would be 320 g/mol.