Decomposition is a chemical reaction that breaks the reactant into two or more products. Moles of nitrogen gas 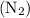 in the cylinder is 1.63 moles.
in the cylinder is 1.63 moles.
The ideal gas equation states the relation of the hypothetical ideal gas according to the pressure, volume, temperature and moles of the gas. It is given by,
Where,
Pressure (P) = 2000 kPa
Volume (V) = 2L
Temperature (T) = 295 K
Gas constant (R)= 0.08206
Substituting values in the equation:
Therefore, 1.63 moles are produced.
Learn more about ideal gas equation here: