The pH does not decrease drastically because the HCl reacts with the sodium azide (NaN₃) present in the buffer solution.
Explanation:
The buffer solution is formed by 0.26 moles of the weak acid, hydrazoic acid (HN₃), and by 0.26 moles of sodium azide (NaN₃). The equilibrium reaction of this buffer solution is the following:
HN₃(aq) + H₂O(l) ⇄ N₃⁻(aq) + H₃O⁺(aq)
The pH of this solution is:
![pH = pka + log(\frac{[N_{3}^{-}]}{[HN_{3}]}) = -log(2.5 \cdot 10^{-5}) + log(\frac{0.26 mol/1 L}{0.26 mol/1 L}) = 4.60](/tpl/images/0673/9468/07a5f.png)
When 0.05 moles of HCl is added to the buffer solution, the following reaction takes place:
H₃O⁺(aq) + N₃⁻(aq) ⇄ HN₃(aq) + H₂O(l)
The number of moles of NaN₃ after the reaction with HCl is:
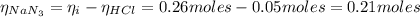
Now, the number of moles of HN₃ is:
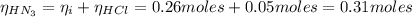
Then, the pH of the buffer solution after the addition of HCl is:
![pH = pka + log(\frac{[N_{3}^{-}]}{[HN_{3}]}) = -log(2.5 \cdot 10^{-5}) + log(\frac{0.21 mol/V_{T}}{0.31 mol/V_{T}}) = 4.43](/tpl/images/0673/9468/7fc10.png)
The pH of the buffer solution does not decrease drastically, it is 4.60 before the addition of HCl and 4.43 after the addition of HCl.
Therefore, the pH does not decrease drastically because the HCl reacts with the sodium azide (NaN₃) present in the buffer solution.
I hope it helps you!