Answer:
A) Considering the solution of molality, molarity and density, the molality of the glycerol solution is 0.029 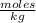 .
.
Molality is a measure of concentration that indicates the ratio of the number of moles of any dissolved solute to kilograms of solvent.
The Molality of a solution is determined by the expression:
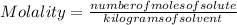
Molality is expressed in units 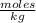 .
.
B) In this case, you know a 2.950×10⁻² M solution of glycerol (C₃H₈O₃) in water is at 20.0∘C. The sample was created by dissolving a sample of C₃H₈O₃ in water and then bringing the volume up to 1 L.
So, being the molarity the number of moles of solute that are dissolved in a certain volume and determined by the following expression:
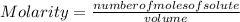
the number of moles of solute can be calculated as:
number of moles of solute= molarity× volume
Then, in this case, the number of moles of solute is calculated as:
number of moles of solute= 2.950×10⁻² M× 1 L
Solving:
number of moles of solute= 2.950×10⁻² moles
On the other side, density is a quantity that allows us to measure the amount of mass in a certain volume of a substance. Then, the expression for the calculation of density is the quotient between the mass of a body and the volume it occupies:
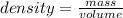
C) Then, the mass can be calculated as:
mass= density×volume
In this case, you know that the volume of water needed was 998.7 mL and the density of water at 20.0∘C is 0.9982 g/mL. So the mass of water can be calculated as:
mass of water= mass of solvent= 0.9982 g/mL× 998.7 mL= 996.9 g= 0.9969 kg
D) Finally, the molality is calculated as:
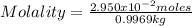
Solving:
Molality= 0.029 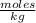
In summary, the molality of the glycerol solution is 0.029 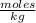 .
.