Answer:
see below.
Step-by-step explanation:
To solve this problem, we can use the conservation of energy and conservation of momentum principles.
Conservation of energy:
The total initial energy is the rest energy of the proton and neutron, which is given by:
Ei = (mp + mn)c^2
where mp and mn are the masses of the proton and neutron, respectively, and c is the speed of light.
The total final energy is the rest energy of the deuteron plus the energy of the gamma ray, which is given by:
Ef = (md)c^2 + Eg
where md is the mass of the deuteron and Eg is the energy of the gamma ray.
According to the conservation of energy principle, the initial energy and final energy must be equal, so we have:
Ei = Ef
(mp + mn)c^2 = (md)c^2 + Eg
Conservation of momentum:
The total initial momentum is zero because the proton and neutron are at rest. The total final momentum is the momentum of the deuteron and the momentum of the gamma ray. Since the gamma ray is massless, its momentum is given by:
pg = Eg/c
where pg is the momentum of the gamma ray.
According to the conservation of momentum principle, the total final momentum must be equal to zero, so we have:
0 = pd + pg
where pd is the momentum of the deuteron.
Solving for md and pd:
From the conservation of energy equation, we can solve for md:
md = (mp + mn - Eg/c^2)/c^2
Substituting this expression into the conservation of momentum equation, we get:
pd = -pg = -Eg/c
Substituting the given values, we have:
mp = 1.6726 × 10^-27 kg mn = 1.6749 × 10^-27 kg Eg = 2.2 × 10^6 eV = 3.52 × 10^-13 J
Using c = 2.998 × 10^8 m/s, we get:
md = (1.6726 × 10^-27 kg + 1.6749 × 10^-27 kg - 3.52 × 10^-13 J/(2.998 × 10^8 m/s)^2)/(2.998 × 10^8 m/s)^2 = 3.3435 × 10^-27 kg
pd = -Eg/c = -(3.52 × 10^-13 J)/(2.998 × 10^8 m/s) = -1.1723 × 10^-21 kg·m/s
Therefore, the mass of the deuteron is 3.3435 × 10^-27 kg, and its momentum is -1.1723 × 10^-21 kg·m/s.
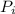 : is the initial pressure = 5.79 atm
: is the initial pressure = 5.79 atm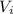 : is the initial volume = 420 cm³
: is the initial volume = 420 cm³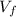 : is the final volume = 1450 cm³
: is the final volume = 1450 cm³