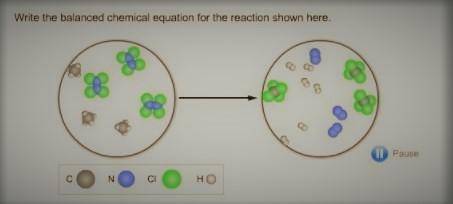
The balanced chemical equation for the reaction is:
3CH₄ + 3 N₂Cl₄ → 3 CCl₄ + 3N₂ + 6H₂
What is a balanced chemical equation?A balanced chemical equation is a chemical reaction that has equal numbers of moles of chemical substances both in the reactant and the product side.
From the missing image that is attached below, we can see that the picture depicts that we have:
The reactant side:
3 molecules of CH₄ and 3 molecules of N₂Cl₄The product side:
3 molecules of CCl₄, 3 molecules of N₂ and 6 molecules of H₂Therefore, the balanced chemical equation for the reaction is:
3CH₄ + 3 N₂Cl₄ → 3 CCl₄ + 3N₂ + 6H₂
Learn more about Balanced chemical reactions here: