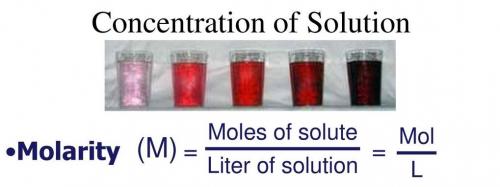
Answer:
AStep-by-step explanation:
The input force is 50 N. But it will not create not any change. No mechanical advantage is observed.
Calcium (Ca)(On the periodic table, ionization energy increases as you go up and to the right of the periodic table)

It will provide an instant answer!
