The net ionic equation is
2Ag^+(aq) + SO4^2-(aq) → Ag2SO4 (s)
explanationwrite the chemical equation for the reaction
2AgNO3 (aq) + Na2SO4 (aq)→ Ag2SO4 (s) + 2 NaNO3 (aq)
write the ionic equation
2Ag^+(aq) + 2 NO3^- (aq) +2 Na^+(aq) + SO4^2-(aq) → Ag2SO4 (s) +2 Na^+(aq) + 2NO3^-
cancel the spectator ions (NO3^- and Na^+)
the net ionic equation is therefore
= 2Ag ^+(aq) + SO4^2-(aq) → Ag2SO4(s)
The net ionic equation for the reaction for the reaction between AgNO3(aq) and Na2SO4(aq) is
2Ag ^+(aq) + SO4^2-(aq) → Ag2SO4 (s)
Explanation
Step 1: write a balanced molecular equation between AgNO3 and Na2SO4
2AgNO3(aq) + Na2SO4 (aq) → Ag2So4(s) +2NaNO3 (aq)
Step 2: Break all the soluble electrolytes into their ions
Ag^+(aq) +NO3^-(aq) + 2Na^+(aq) +SO4^2- (aq)→
Ag2SO4(s) + 2Na^+(aq) +NO3^-(aq)
Step 3: cancel the spectator ions( ions that exist in the same form on both reactant and product side of equation above)
= 2 Na^+ and NO^-
Step 4: write down the net equation
Ag^+(aq) +SO4^2- (aq)→ Ag2SO4 (s)
Explanation:
1) ZnBr₂ (aq) + AgNO₃ (aq)
Chemical equation:
ZnBr₂ (aq) + AgNO₃ (aq) →Zn(NO₃)₂(aq) + AgBr(s)
Balanced chemical equation:
ZnBr₂ (aq) + 2AgNO₃ (aq) →Zn(NO₃)₂(aq) + 2AgBr(s)
Ionic equation:
Zn²⁺(aq) + Br₂²⁻ (aq) + 2Ag⁺ (aq)+ 2NO⁻₃ (aq) → Zn²⁺(aq) +(NO₃)₂²⁻(aq) + 2AgBr(s)
Net ionic equation:
Br₂²⁻ (aq) + 2Ag⁺ (aq) → 2AgBr(s)
The Zn²⁺((aq) and NO⁻₃ (aq) are spectator ions that's why these are not written in net ionic equation. The AgBr can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
2) Ca(OH)₂ (aq) + Na₂SO₄ (aq)
Chemical equation:
Ca(OH)₂ (aq) + Na₂SO₄ (aq) → CaSO₄(s) + NaOH(aq)
Balanced chemical equation:
Ca(OH)₂ (aq) + Na₂SO₄ (aq) → CaSO₄(s) + 2NaOH(aq)
Ionic equation:
Ca²⁺(aq) + OH₂²⁻ (aq) + 2Na⁺(aq) + SO₄²⁻ (aq) → CaSO₄(s) + 2Na⁺(aq) + 2OH⁻ (aq)
Net ionic equation:
Ca²⁺(aq) + SO₄²⁻ (aq) → CaSO₄(s)
The OH⁻ ((aq) and Na⁺ (aq) are spectator ions that's why these are not written in net ionic equation. The CaSO₄ can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
3) Al(NO₃)₃ (aq) + Na₃PO₄ (aq)
Chemical equation:
Al(NO₃)₃ (aq) + Na₃PO₄ (aq) → Al(PO₄)(s) + NaNO₃ (aq)
Balanced chemical equation:
Al(NO₃)₃ (aq) + Na₃PO₄ (aq) → Al(PO₄)(s) + 3NaNO₃ (aq)
Ionic equation:
Al³⁺(aq) + 3NO⁻₃ (aq) + 3Na⁺(aq) + PO₄³⁻ (aq) → Al(PO₄)(s) + 3Na⁺(aq) + NO⁻₃ (aq)
Net ionic equation:
Al³⁺(aq) + PO₄³⁻ (aq) → Al(PO₄)(s)
The Na⁺((aq) and NO⁻₃ (aq) are spectator ions that's why these are not written in net ionic equation. The Al(PO₄) can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
4) FeSO₄ (aq) + Ba(OH)₂ (aq)
Chemical equation:
FeSO₄ (aq) + Ba(OH)₂ (aq) → BaSO₄(s) + Fe(OH)₂(aq)
The equation is already balanced.
Ionic equation:
Fe²⁺(aq) + SO₄²⁻ (aq) + Ba²⁺(aq) + 2OH⁻ (aq) → BaSO₄(s) + Fe²⁺(aq) + 2OH⁻(aq)
Net ionic equation:
SO₄²⁻ (aq) + Ba²⁺(aq) → BaSO₄(s)
The Fe²⁺ (aq) and OH⁻ (aq) are spectator ions that's why these are not written in net ionic equation. The BaSO₄ can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
Explanation:
1) ZnBr₂ (aq) + AgNO₃ (aq)
Chemical equation:
ZnBr₂ (aq) + AgNO₃ (aq) →Zn(NO₃)₂(aq) + AgBr(s)
Balanced chemical equation:
ZnBr₂ (aq) + 2AgNO₃ (aq) →Zn(NO₃)₂(aq) + 2AgBr(s)
Ionic equation:
Zn²⁺(aq) + Br₂²⁻ (aq) + 2Ag⁺ (aq)+ 2NO⁻₃ (aq) → Zn²⁺(aq) +(NO₃)₂²⁻(aq) + 2AgBr(s)
Net ionic equation:
Br₂²⁻ (aq) + 2Ag⁺ (aq) → 2AgBr(s)
The Zn²⁺((aq) and NO⁻₃ (aq) are spectator ions that's why these are not written in net ionic equation. The AgBr can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
2) Ca(OH)₂ (aq) + Na₂SO₄ (aq)
Chemical equation:
Ca(OH)₂ (aq) + Na₂SO₄ (aq) → CaSO₄(s) + NaOH(aq)
Balanced chemical equation:
Ca(OH)₂ (aq) + Na₂SO₄ (aq) → CaSO₄(s) + 2NaOH(aq)
Ionic equation:
Ca²⁺(aq) + OH₂²⁻ (aq) + 2Na⁺(aq) + SO₄²⁻ (aq) → CaSO₄(s) + 2Na⁺(aq) + 2OH⁻ (aq)
Net ionic equation:
Ca²⁺(aq) + SO₄²⁻ (aq) → CaSO₄(s)
The OH⁻ ((aq) and Na⁺ (aq) are spectator ions that's why these are not written in net ionic equation. The CaSO₄ can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
3) Al(NO₃)₃ (aq) + Na₃PO₄ (aq)
Chemical equation:
Al(NO₃)₃ (aq) + Na₃PO₄ (aq) → Al(PO₄)(s) + NaNO₃ (aq)
Balanced chemical equation:
Al(NO₃)₃ (aq) + Na₃PO₄ (aq) → Al(PO₄)(s) + 3NaNO₃ (aq)
Ionic equation:
Al³⁺(aq) + 3NO⁻₃ (aq) + 3Na⁺(aq) + PO₄³⁻ (aq) → Al(PO₄)(s) + 3Na⁺(aq) + NO⁻₃ (aq)
Net ionic equation:
Al³⁺(aq) + PO₄³⁻ (aq) → Al(PO₄)(s)
The Na⁺((aq) and NO⁻₃ (aq) are spectator ions that's why these are not written in net ionic equation. The Al(PO₄) can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
4) FeSO₄ (aq) + Ba(OH)₂ (aq)
Chemical equation:
FeSO₄ (aq) + Ba(OH)₂ (aq) → BaSO₄(s) + Fe(OH)₂(aq)
The equation is already balanced.
Ionic equation:
Fe²⁺(aq) + SO₄²⁻ (aq) + Ba²⁺(aq) + 2OH⁻ (aq) → BaSO₄(s) + Fe²⁺(aq) + 2OH⁻(aq)
Net ionic equation:
SO₄²⁻ (aq) + Ba²⁺(aq) → BaSO₄(s)
The Fe²⁺ (aq) and OH⁻ (aq) are spectator ions that's why these are not written in net ionic equation. The BaSO₄ can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
The net ionic equation for the reaction for the reaction between AgNO3(aq) and Na2SO4(aq) is
2Ag ^+(aq) + SO4^2-(aq) → Ag2SO4 (s)
Explanation
Step 1: write a balanced molecular equation between AgNO3 and Na2SO4
2AgNO3(aq) + Na2SO4 (aq) → Ag2So4(s) +2NaNO3 (aq)
Step 2: Break all the soluble electrolytes into their ions
Ag^+(aq) +NO3^-(aq) + 2Na^+(aq) +SO4^2- (aq)→
Ag2SO4(s) + 2Na^+(aq) +NO3^-(aq)
Step 3: cancel the spectator ions( ions that exist in the same form on both reactant and product side of equation above)
= 2 Na^+ and NO^-
Step 4: write down the net equation
Ag^+(aq) +SO4^2- (aq)→ Ag2SO4 (s)
Answer:
AStep-by-step explanation:
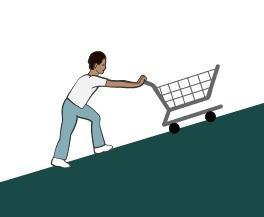
The input force is 50 N. But it will not create not any change. No mechanical advantage is observed.
 1
1 Answer:
52.6 gramStep-by-step explanation:
It is clear by the equation 2(27+3×35.5)= 267 gm of AlCl3 reacts with 6× 80 = 480 gm of Br2 . So 29.2 gm reacts = 480× 29.2/267= 52.6 gm
glycoproteins
Explanation:
A positive reaction for Molisch's test is given by almost all carbohydrates (exceptions include tetroses & trioses). It can be noted that even some glycoproteins and nucleic acids give positive results for this test (since they tend to undergo hydrolysis when exposed to strong mineral acids and form monosaccharides).
Answer:
Taking into accoun the ideal gas law, The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.
An ideal gas is a theoretical gas that is considered to be composed of point particles that move randomly and do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas. The universal constant of ideal gases R has the same value for all gaseous substances.
Explanation:
In this case, you know:
P= 0.884 atm
V= ?
n= 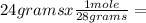 0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
0.857 moles (where 28 g/mole is the molar mass of N₂, that is, the amount of mass that the substance contains in one mole.)
R=0.082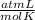
T= 328 K
Replacing in the ideal gas law:
0.884 atm×V= 0.857 moles× 0.082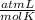 ×328 K
×328 K
Solving:
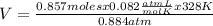
V= 26.07 L
The volume of a container that contains 24.0 grams of N2 gas at 328K and 0.884 atm is 26.07 L.

It will provide an instant answer!
