Bombarding sodium-23 with a proton produces nuclide X and a neutron. What is nuclide X?

neon
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
The isotope P has a half-life of 14.3 days. If a sample originally contained 1.00 g of P,
how much was left after 43 days?
0 days >>> 1.00g
14,3 days >>> 1.00g :2 = 0,5g
(14.3+14.3) days >>> 0.5g : 2 = 0.25g
(14.3+14.3+14.3) days >>> 0.25g : 2 = 0.125g
0.125g
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Identify X in the reaction
below.
U + C → Cf + X
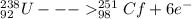
6 electrons (so it's beta⁻)
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
A .20 gram sample of C-14 was allowed to decay for 3 half-lives. What mass
of the sample will remain? Carbon-14 has a half life of 5730
years.
at the beginning >>> 0.20g
1 half-live >>> 0.20g : 2 = 0.10g
2 half-live >>> 0.10g : 2 = 0.05g
3 half-live >>> 0.05g : 2 = 0.025g
0.025g
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
The isotope Cu has a half-life of 30 s. If a sample originally contained 48 mg of Cu,
how much time passed before the amount fell to 3 mg?
0 s (at the beginning) >>> 48mg
30s >>>>>>>>>>>>>>> 48mg : 2 = 24mg
(30+30)s >>>>>>>>>>> 24mg : 2 = 12mg
(30+30+30)s >>>>>>>> 12mg : 2 = 6mg
(30+30+30+30)s >>>>> 6mg : 2 = 3mg
30+30+30+30 = 120s
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
What radionuclide decays to Fe-56 by beta emission?

⁵⁶₂₇Co
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
The Cf to Cf conversion is accompanied by __________.
Cf to Cf ???? maybe...mistake?