Problem 1-
The acceleration = 5 m/s²
Problem 2 -
The acceleration = 3.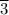 m/s²
m/s²
Problem 3 -
The acceleration = 2.5 m/s²
Problem 4-
The force applied = 7.5 N
Problem 5-
The mass of the object, = 0.5 kg.
Explanation:
Problem 1-
The mass of the ball, m = 0.5 kg
The force (applied) with which it was hit, F = 2.5 N
The acceleration, a = Force/Mass = 2.5 N/(0.5 kg) = 5 m/s²
The acceleration = 5 m/s²
Problem 2 -
The mass of the box, m = 15 kg
The force (applied) with which it was hit, F = 50 N
The acceleration, a = Force/Mass = 50 N/(15 kg) = 10/3 = 3.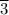 m/s²
m/s²
The acceleration = 3.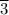 m/s²
m/s²
Problem 3 -
The mass of the object, m = 2.0 kg
The force (applied) with which it was hit, F = 5.0 N
The acceleration, a = Force/Mass = 5.0 N/(2.0 kg) = 2.5 m/s²
The acceleration = 2.5 m/s²
Problem 4-
The mass of the object, m = 3.0 kg
The acceleration, a, of the object = 2.5 m/s²
The force applied to it, F = Mass × Acceleration = 3.0 kg × 2.5 m/s² = 7.5 N
The force applied = 7.5 N
Problem 5-
The acceleration, a, of the object = 12 m/s²
The force applied, F = 6.0 N
The mass of the object, m = Force/Acceleration = 6.0 N/(12 m/s²) = 0.5 kg
The mass of the object, = 0.5 kg.