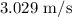
Explanation:
m = Mass of pendulum = 1 kg
L = Length of pendulum = 2 m
g = Acceleration due to gravity = 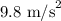
h = Height of the pendulum = 0.468 m
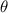 = Angle of deflection =
= Angle of deflection = 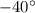
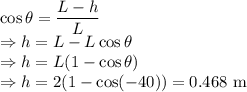
The energy balance of the pendulum is as follows
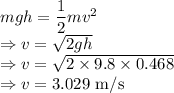
The maximum velocity of this pendulum is 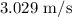 .
.